An inactivated vaccine to control the current H9N2 low pathogenic avian influenza in Korea
- PMID: 18296890
- PMCID: PMC2839114
- DOI: 10.4142/jvs.2008.9.1.67
An inactivated vaccine to control the current H9N2 low pathogenic avian influenza in Korea
Abstract
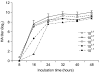
The H9N2 subtype low pathogenic avian influenza is one of the most prevalent avian diseases worldwide, and was first documented in 1996 in Korea. This disease caused serious economic loss in Korea's poultry industry. In order to develop an oil-based inactivated vaccine, a virus that had been isolated in 2001 (A/chicken/Korea/01310/2001) was selected based on its pathogenic, antigenic, and genetic properties. However, in animal experiments, the efficacy of the vaccine was found to be very low without concentration of the antigen (2 7 to 2 10 hemagglutinin unit). In order to overcome the low productivity, we passaged the vaccine candidate virus to chicken eggs. After the 20th passage, the virus was approximately ten times more productive compared with the parent virus. For the most part, the passaged virus maintained the hemagglutinin cleavage site amino acid motif (PATSGR/GLF) and had only three amino acid changes (T133N, V216G, E439D, H3 numbering) in the hemagglutinin molecule, as well as 18 amino acid deletions (55-72) and one amino acid change (E54D) in the NA stalk region. The amino acid changes did not significantly affect the antigenicity of the vaccine virus when tested by hemagglutination inhibition assay. Though not complete, the vaccine produced after the 20th passage of the virus (01310 CE20) showed good protection against a homologous and recent Korean isolate (A/chicken/Korea/Q30/2004) in specific pathogen- free chickens. The vaccine developed in this study would be helpful for controlling the H9N2 LPAI in Korea.
Figures
References
-
- Asaoka N, Tanaka Y, Sakai T, Fujii Y, Ohuchi R, Ohuchi M. Low growth ability of recent influenza clinical isolates in MDCK cells is due to their low receptor binding affinities. Microbes Infect. 2006;8:511–519. - PubMed
-
- Baigent SJ, McCauley JW. Glycosylation of haemagglutinin and stalk-length of neuraminidase combine to regulate the growth of avian influenza viruses in tissue culture. Virus Res. 2001;79:177–185. - PubMed
-
- Banet-Noach C, Perk S, Simanov L, Grebenyuk N, Rozenblut E, Pokamunski S, Pirak M, Tendler Y, Panshin A. H9N2 influenza viruses from Israeli poultry: a five-year outbreak. Avian Dis. 2007;51(Suppl 1):290–296. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical