CD54 is a surrogate marker of antigen presenting cell activation
- PMID: 18297282
- PMCID: PMC11030627
- DOI: 10.1007/s00262-008-0474-9
CD54 is a surrogate marker of antigen presenting cell activation
Abstract
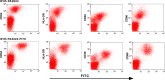
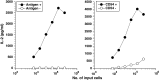
There is no single universally accepted hallmark of antigen presenting cell (APC) activation. Instead a variety of methods are used to identify APCs and assess their activation state. These activation measures include phenotypic methods [e.g., assessing the increased expression of surface markers such as major histocompatability (MHC) class II] and functional assays (e.g., evaluating the enhanced ability to take up and process antigen, or stimulate naïve T cells). Sipuleucel-T is an investigational autologous active cellular immunotherapy product designed to stimulate a T cell immune response against human prostatic acid phosphatase (PAP), an antigen highly expressed in prostate tissue. Sipuleucel-T consists of peripheral blood mononuclear cells (PBMCs), including activated APCs displaying epitopes of PAP. In order to develop a robust reproducible potency assay that is not hampered by MHC restriction we have developed a method to simply assess the biological activation of antigen presenting cells (APCs). In the course of sipuleucel-T characterization, we analyzed various phenotypic and functional parameters to define the activation state of APCs obtained from peripheral blood. Flow cytometric assays revealed that CD54+ cells are responsible for antigen uptake, and that expression of CD54 predominantly localizes to APCs. Costimulation, as measured by an allogeneic mixed lymphocytic reaction (alloMLR) assay, showed that activity was restricted to the CD54+ cell population. Similarly, CD54+ cells harbor all of the PAP-specific antigen presentation activity, as assayed using a PAP-specific HLA-DRbeta1-restricted T cell hybridoma. Finally we show that CD54 expression is substantially and consistently upregulated on APCs during culture with a GM-CSF fusion protein, and that this upregulation activity can be quantified. Thus these data support the use of CD54 upregulation as a surrogate for assessing human APC activation and validates its utility as a potency measure of sipuleucel-T.
Figures




References
-
- Burch PA, Breen JK, Buckner JC, Gastineau DA, Kaur JA, Laus RL, Padley DJ, Peshwa MV, Pitot HC, Richardson RL, Smits BJ, Sopapan P, Strang G, Valone FH, Vuk-Pavlovic S. Priming tissue-specific cellular immunity in a phase I trial of autologous dendritic cells for prostate cancer. Clin Cancer Res. 2000;6:2175–2182. - PubMed
-
- Burch PA, Groghan GA, Gastineau DA, Jones LA, Kaur JS, Kylstra JW, Richardson RL, Valone FH, Vuk-Pavlovic S. Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-induced prostate cancer: a phase 2 trial. Prostate. 2004;60:197–204. doi: 10.1002/pros.20040. - DOI - PubMed
Online references
-
- From a presentation at the Cellular, Tissue and Gene Therapies Advisory Committee meeting. January 2007 posting date. Gavin D. Perspectives on potency assays for complex biological products. Food and Drug Administration, Center for Biologics Research and Evaluation, Office of Cellular, Tissue and gene Therapy. http://www.fda.gov/cber/genetherapy/cmc012807dg.htm
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

