The PIX-GIT complex: a G protein signaling cassette in control of cell shape
- PMID: 18299239
- PMCID: PMC2394276
- DOI: 10.1016/j.semcdb.2008.01.002
The PIX-GIT complex: a G protein signaling cassette in control of cell shape
Abstract
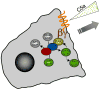
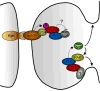
Arf and Rho GTP-binding proteins coordinately regulate membrane dynamics and cytoskeletal rearrangements. The Cdc42/Rac guanine nucleotide exchange factor PIX and the Arf GTPase-activating protein GIT form a stable complex in cells. The PIX-GIT complex functions to integrate signaling among Arf, Cdc42, and Rac proteins in response to cues emanating from integrins, heterotrimeric G proteins, receptor tyrosine kinases, and cell-cell interactions. A concept that emerges from the literature is that the PIX-GIT complex serves as a cassette to elicit changes in cell shape essential for polarized cell responses in a wide range of biological contexts.
Figures




Similar articles
-
[The GIT-PIX protein complex: a hub to ARF and Rac/Cdc42 GTPases].Med Sci (Paris). 2005 Oct;21(10):849-53. doi: 10.1051/medsci/20052110849. Med Sci (Paris). 2005. PMID: 16197902 Review. French.
-
Expanding functions of GIT Arf GTPase-activating proteins, PIX Rho guanine nucleotide exchange factors and GIT-PIX complexes.J Cell Sci. 2016 May 15;129(10):1963-74. doi: 10.1242/jcs.179465. J Cell Sci. 2016. PMID: 27182061 Free PMC article. Review.
-
The GIT/PIX complex: an oligomeric assembly of GIT family ARF GTPase-activating proteins and PIX family Rac1/Cdc42 guanine nucleotide exchange factors.Cell Signal. 2004 Sep;16(9):1001-11. doi: 10.1016/j.cellsig.2004.02.002. Cell Signal. 2004. PMID: 15212761
-
Role of phospholipase Cgamma1 in cell spreading requires association with a beta-Pix/GIT1-containing complex, leading to activation of Cdc42 and Rac1.Mol Cell Biol. 2007 Aug;27(16):5790-805. doi: 10.1128/MCB.00778-07. Epub 2007 Jun 11. Mol Cell Biol. 2007. PMID: 17562871 Free PMC article.
-
Structures of dimeric GIT1 and trimeric beta-PIX and implications for GIT-PIX complex assembly.J Mol Biol. 2009 Feb 20;386(2):280-9. doi: 10.1016/j.jmb.2008.12.050. Epub 2008 Dec 30. J Mol Biol. 2009. PMID: 19136011
Cited by
-
The adaptor protein and Arf GTPase-activating protein Cat-1/Git-1 is required for cellular transformation.J Biol Chem. 2012 Sep 7;287(37):31462-70. doi: 10.1074/jbc.M112.353615. Epub 2012 Jul 17. J Biol Chem. 2012. PMID: 22807447 Free PMC article.
-
Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma.Cell. 2008 Nov 28;135(5):865-78. doi: 10.1016/j.cell.2008.09.045. Cell. 2008. PMID: 19041750 Free PMC article.
-
Prostaglandins in cancer cell adhesion, migration, and invasion.Int J Cell Biol. 2012;2012:723419. doi: 10.1155/2012/723419. Epub 2012 Feb 29. Int J Cell Biol. 2012. PMID: 22505934 Free PMC article.
-
The focal adhesion-associated proteins DOCK5 and GIT2 comprise a rheostat in control of epithelial invasion.Oncogene. 2017 Mar 30;36(13):1816-1828. doi: 10.1038/onc.2016.345. Epub 2016 Sep 26. Oncogene. 2017. PMID: 27669437 Free PMC article.
-
The Drosophila tumour suppressor Lgl and Vap33 activate the Hippo pathway through a dual mechanism.J Cell Sci. 2024 Feb 15;137(4):jcs261917. doi: 10.1242/jcs.261917. Epub 2024 Feb 16. J Cell Sci. 2024. PMID: 38240353 Free PMC article.
References
-
- Hall A. Rho GTPases and the actin cytoskeleton. Science (New York, NY) 1998;279(5350):509–14. - PubMed
-
- Settleman J. Getting in shape with Rho. Nature Cell Biology. 2000;2:E7–9. - PubMed
-
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004 Jan 23;116(2):167–79. - PubMed
-
- D'Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006 May;7(5):347–58. - PubMed
-
- Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81(1):153–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

