Expression of the type III TGF-beta receptor is negatively regulated by TGF-beta
- PMID: 18299279
- PMCID: PMC2902381
- DOI: 10.1093/carcin/bgn049
Expression of the type III TGF-beta receptor is negatively regulated by TGF-beta
Abstract
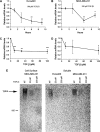
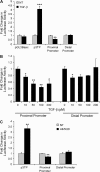
The type III transforming growth factor-beta receptor (TbetaRIII or betaglycan) is a ubiquitously expressed transforming growth factor-beta (TGF-beta) superfamily coreceptor with essential roles in embryonic development. Recent studies have defined a role for TbetaRIII in the pathogenesis of human cancers, with frequent loss of TbetaRIII expression at the message and protein level. Mechanisms for the loss of TbetaRIII expression remain to be fully defined. Advanced human cancers often have elevated circulating levels of TGF-beta1. Here, we define a specific role for TGF-beta1 in negatively regulating TbetaRIII at the message level in breast and ovarian cancer models. TGF-beta1 decreased TbetaRIII message and protein levels in ovarian (Ovca420) and breast cancer (MDA-MB-231) cell lines in both a dose- and time-dependent manner. TGF-beta1-mediated TbetaRIII repression is mediated by the type I TGF-beta receptor/Smad2/3 pathway as the activin receptor-like kinase 5 (ALK5) inhibitor, SB431542, abrogated this effect, while the expression of constitutively active ALK5 was sufficient to repress TbetaRIII expression. Mechanistically, TGF-beta1 does not affect TbetaRIII messenger RNA (mRNA) stability, but instead directly regulates the TbetaRIII promoter. We define alternative promoters for the TGFBR3 gene, a distal and proximal promoter. Although both promoters are active, only the proximal promoter was responsive and negatively regulated by TGF-beta1 and constitutively active ALK5. Taken together, these studies define TGF-beta1-mediated downregulation of TbetaRIII mRNA expression through effects on the ALK5/Smad2/3 pathway on the TGFBR3 gene proximal promoter as a potential mechanism for decreased TbetaRIII expression in human cancers.
Figures


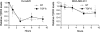

References
-
- Elliott RL, et al. Role of transforming growth factor Beta in human cancer. J. Clin. Oncol. 2005;23:2078–2093. - PubMed
-
- Wrana JL, et al. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347. - PubMed
-
- Lopez-Casillas F, et al. Betaglycan presents ligand to the TGF beta signaling receptor. Cell. 1993;73:1435–1444. - PubMed
-
- Lewis KA, et al. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature. 2000;404:411–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

