IKK/NF-kappaB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis
- PMID: 18299349
- PMCID: PMC2265568
- DOI: 10.1083/jcb.200707179
IKK/NF-kappaB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis
Abstract
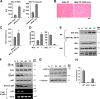
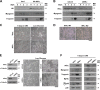
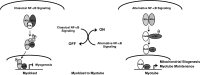
Nuclear factor kappaB (NF-kappaB) is involved in multiple skeletal muscle disorders, but how it functions in differentiation remains elusive given that both anti- and promyogenic activities have been described. In this study, we resolve this by showing that myogenesis is controlled by opposing NF-kappaB signaling pathways. We find that myogenesis is enhanced in MyoD-expressing fibroblasts deficient in classical pathway components RelA/p65, inhibitor of kappaB kinase beta (IKKbeta), or IKKgamma. Similar increases occur in myoblasts lacking RelA/p65 or IKKbeta, and muscles from RelA/p65 or IKKbeta mutant mice also contain higher fiber numbers. Moreover, we show that during differentiation, classical NF-kappaB signaling decreases, whereas the induction of alternative members IKKalpha, RelB, and p52 occurs late in myogenesis. Myotube formation does not require alternative signaling, but it is important for myotube maintenance in response to metabolic stress. Furthermore, overexpression or knockdown of IKKalpha regulates mitochondrial content and function, suggesting that alternative signaling stimulates mitochondrial biogenesis. Together, these data reveal a unique IKK/NF-kappaB signaling switch that functions to both inhibit differentiation and promote myotube homeostasis.
Figures







References
-
- Acharyya, S., M.E. Butchbach, Z. Sahenk, H. Wang, M. Saji, M. Carathers, M.D. Ringel, R.J. Skipworth, K.C. Fearon, M.A. Hollingsworth, et al. 2005. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell. 8:421–432. - PubMed
-
- Acharyya, S., S.A. Villalta, N. Bakkar, T. Bupha-Intr, P.M. Janssen, M. Carathers, Z.W. Li, A.A. Beg, S. Ghosh, Z. Sahenk, et al. 2007. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J. Clin. Invest. 117:889–901. - PMC - PubMed
-
- Anest, V., J.L. Hanson, P.C. Cogswell, K.A. Steinbrecher, B.D. Strahl, and A.S. Baldwin. 2003. A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature. 423:659–663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

