The somatodendritic endosomal regulator NEEP21 facilitates axonal targeting of L1/NgCAM
- PMID: 18299352
- PMCID: PMC2265569
- DOI: 10.1083/jcb.200707143
The somatodendritic endosomal regulator NEEP21 facilitates axonal targeting of L1/NgCAM
Abstract
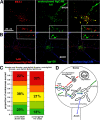
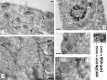
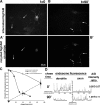
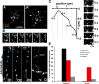
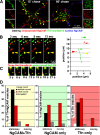
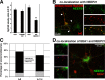
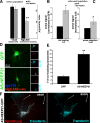
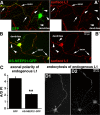
Correct targeting of proteins to axons and dendrites is crucial for neuronal function. We showed previously that axonal accumulation of the cell adhesion molecule L1/neuron-glia cell adhesion molecule (NgCAM) depends on endocytosis (Wisco, D., E.D. Anderson, M.C. Chang, C. Norden, T. Boiko, H. Folsch, and B. Winckler. 2003. J. Cell Biol. 162:1317-1328). Two endocytosis-dependent pathways to the axon have been proposed: transcytosis and selective retrieval/retention. We show here that axonal accumulation of L1/NgCAM occurs via nondegradative somatodendritic endosomes and subsequent anterograde axonal transport, which is consistent with transcytosis. Additionally, we identify the neuronal-specific endosomal protein NEEP21 (neuron-enriched endosomal protein of 21 kD) as a regulator of L1/NgCAM sorting in somatodendritic endosomes. Down-regulation of NEEP21 leads to missorting of L1/NgCAM to the somatodendritic surface as well as to lysosomes. Importantly, the axonal accumulation of endogenous L1 in young neurons is also sensitive to NEEP21 depletion. We propose that small endosomal carriers derived from somatodendritic recycling endosomes can serve to redistribute a distinct set of membrane proteins from dendrites to axons.
Figures

References
-
- Alberi, S., B. Boda, P. Steiner, I. Nikonenko, H. Hirling, and D. Muller. 2005. The endosomal protein NEEP21 regulates AMPA receptor-mediated synaptic transmission and plasticity in the hippocampus. Mol. Cell. Neurosci. 29:313–319. - PubMed
-
- Alberts, P., and T. Galli. 2003. The cell outgrowth secretory endosome (COSE): a specialized compartment involved in neuronal morphogenesis. Biol. Cell. 95:419–424. - PubMed
-
- Alberts, P., R. Rudge, I. Hinners, A. Muzerelle, S. Martinez-Arca, T. Irinopoulou, V. Marthiens, S. Tooze, F. Rathjen, P. Gaspar, and T. Galli. 2003. Cross talk between tetanus neurotoxin-insensitive vesicle-associated membrane protein-mediated transport and L1-mediated adhesion. Mol. Biol. Cell. 14:4207–4220. - PMC - PubMed
-
- Brown, T.C., I.C. Tran, D.S. Backos, and J.A. Esteban. 2005. NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron. 45:81–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

