Effect of polysorbate 80 on oritavancin binding to plastic surfaces: implications for susceptibility testing
- PMID: 18299406
- PMCID: PMC2346630
- DOI: 10.1128/AAC.01513-07
Effect of polysorbate 80 on oritavancin binding to plastic surfaces: implications for susceptibility testing
Abstract
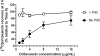
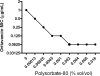
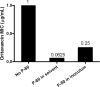
Oritavancin, a semisynthetic lipoglycopeptide with activity against gram-positive bacteria, has multiple mechanisms of action, including the inhibition of cell wall synthesis and the perturbation of the membrane potential. Approved guidelines for broth microdilution MIC assays with dalbavancin, another lipoglycopeptide, require inclusion of 0.002% polysorbate 80. To investigate the potential impact of polysorbate 80 on oritavancin susceptibility assays, we quantified the recovery of [(14)C]oritavancin from susceptibility assay plates with and without polysorbate 80 and examined the effect of the presence of polysorbate 80 on the oritavancin MICs for 301 clinical isolates from the genera Staphylococcus, Enterococcus, and Streptococcus. In the absence of polysorbate 80, [(14)C]oritavancin was rapidly lost from solution in susceptibility assay test plates: 9% of the input drug was recovered in broth at 1 h when [(14)C]oritavancin was tested at 1 mug/ml. Furthermore, proportionately greater losses were observed at lower oritavancin concentrations, suggesting saturable binding of oritavancin to surfaces. The inclusion of 0.002% polysorbate 80 or 2% lysed horse blood permitted the recovery of 80 to 100% [(14)C]oritavancin at 24 h for all drug concentrations tested. Concordantly, oritavancin MIC(90)s for streptococcal isolates, as determined in medium containing 2% lysed horse blood, were identical with and without polysorbate 80. In stark contrast, polysorbate 80 reduced the oritavancin MIC(90)s by 16- to 32-fold for clinical isolates of enterococci and staphylococci, which are typically cultured without blood. The results presented here provide evidence that the MIC data for oritavancin in the current literature significantly underestimate the potency of oritavancin in vitro. Moreover, the combination of data from MIC and [(14)C]oritavancin recovery studies supports the revision of the oritavancin broth microdilution method to include polysorbate 80 throughout the assay.
Figures
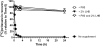
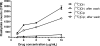
References
-
- Arhin, F. F., I. Sarmiento, A. Belley, G. A. McKay, D. Draghi, P. Grover, D. Sahm, T. R. Parr, Jr., and G. Moeck. 2007. Influence of polysorbate-80 on susceptibility of gram-positive bacteria to oritavancin, abstr. P827. Program Abstr. 17th Eur. Congr. Clin. Microbiol. Infect. Dis. European Society of Clinical Microbiology and Infectious Diseases, Basel, Switzerland.
-
- Arhin, F. F., I. Sarmiento, T. R. Parr, Jr., and G. Moeck. 2007. Effect of polysorbate-80 on oritavancin binding to plastic surfaces—implications for susceptibility testing, abstr. P1112. Program Abstr. 17th Eur. Congr. Clin. Microbiol. Infect. Dis. European Society of Clinical Microbiology and Infectious Diseases, Basel, Switzerland.
-
- Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

