Relationship between cellular response and behavioral variability in bacterial chemotaxis
- PMID: 18299569
- PMCID: PMC2265172
- DOI: 10.1073/pnas.0705463105
Relationship between cellular response and behavioral variability in bacterial chemotaxis
Abstract
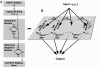
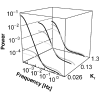
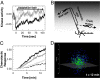
Over the last decades, bacterial chemotaxis in Escherichia coli has emerged as a canonical system for the study of signal transduction. A remarkable feature of this system is the coexistence of a robust adaptive behavior observed at the population level with a large fluctuating behavior in single cells [Korobkova E, Emonet T, Vilar JMG, Shimizu TS, Cluzel P (2004) Nature 428:574-578]. Using a unified stochastic model, we demonstrate that this coexistence is not fortuitous but a direct consequence of the architecture of this adaptive system. The methylation and demethylation cycles that regulate the activity of receptor-kinase complexes are ultrasensitive because they operate outside the region of first-order kinetics. As a result, the receptor-kinase that governs cellular behavior exhibits a sigmoidal activation curve. We propose that the steepness of this kinase activation curve simultaneously controls the behavioral variability in nonstimulated individual bacteria and the duration of the adaptive response to small stimuli. We predict that the fluctuating behavior and the chemotactic response of individual cells both peak within the transition region of this sigmoidal curve. Large-scale simulations of digital bacteria suggest that the chemotaxis network is tuned to simultaneously maximize both the random spread of cells in the absence of nutrients and the cellular response to gradients of attractant. This study highlights a fundamental relation from which the behavioral variability of nonstimulated cells is used to infer the timing of the cellular response to small stimuli.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Pedraza JM, van Oudenaarden A. Science. 2005;307:1965–1969. - PubMed
-
- Austin DW, Allen MS, McCollum JM, Dar RD, Wilgus JR, Sayler GS, Samatova NF, Cox CD, Simpson ML. Nature. 2006;439:608–611. - PubMed
-
- Newman JRS, Ghaemmaghami S, Ihmels J, Breslow DK, Noble M, DeRisi JL, Weissman JS. Nature. 2006;441:840–846. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

