PD-1 deficiency reveals various tissue-specific autoimmunity by H-2b and dose-dependent requirement of H-2g7 for diabetes in NOD mice
- PMID: 18299579
- PMCID: PMC2265199
- DOI: 10.1073/pnas.0710951105
PD-1 deficiency reveals various tissue-specific autoimmunity by H-2b and dose-dependent requirement of H-2g7 for diabetes in NOD mice
Abstract
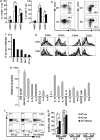
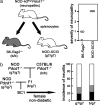
Although many autoimmune diseases are associated with particular HLA/H-2 haplotypes, the mechanisms through which specific HLA/H-2 haplotypes afford autoimmune susceptibility remain enigmatic. Here, we analyzed the effects of the diabetes-associated (H-2(g7)) and an antidiabetogenic (H-2(b)) H-2 haplotypes in NOD mice deficient for programmed cell death-1 (PD-1, Pdcd1), a unique model of type 1 diabetes that confers complete penetrance and rapid onset of the disease. NOD-H2(b/b)Pdcd1(-/-) mice were completely protected from diabetes, confirming that H-2(g7) is indispensable for diabetes even in the absence of PD-1. However, NOD-H2(b/b)Pdcd1(-/-) mice developed autoimmune inflammation in multiple tissues including peripheral nerves, stomachs, and exocrine tissues, demonstrating that autoreactive T cells are generated in the context of H-2(b). These autoreactive T cells damaged target tissues only in the absence of PD-1, confirming that PD-1 deficiency unravels the hidden autoimmune susceptibility of the strain by reducing the threshold of T cell activation. Transfer experiments revealed that CD4 T cells are the effector cells of neuritis, and nerve-infiltrating CD4 T cells are strongly deviated toward Th1. Interestingly, neuritogenic T cells were also generated in the context of H-2(g7), in sharp contrast to the strict requirement of H-2(g7) for diabetes. In addition, 60% of the NOD-H2(b/g7)Pdcd1(-/-) mice developed diabetes, suggesting that H-2(b) does not dominantly suppress diabetes and that H-2(g7) induces diabetes in a dose-dependent fashion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Anderson MS, Bluestone JA. The NOD mouse: A model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. - PubMed
-
- Wicker LS, Todd JA, Peterson LB. Genetic control of autoimmune diabetes in the NOD mouse. Annu Rev Immunol. 1995;13:179–200. - PubMed
-
- Wicker LS, Miller BJ, Fischer PA, Pressey A, Peterson LB. Genetic control of diabetes and insulitis in the non-obese diabetic mouse. Pedigree analysis of a diabetic H-2nod/b heterozygote. J Immunol. 1989;142:781–784. - PubMed
-
- Carrasco-Marin E, Shimizu J, Kanagawa O, Unanue ER. The class II MHC I-Ag7 molecules from non-obese diabetic mice are poor peptide binders. J Immunol. 1996;156:450–458. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

