Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin
- PMID: 18299583
- PMCID: PMC2265195
- DOI: 10.1073/pnas.0708930105
Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin
Abstract
The mechanism of action of the antiepileptic and antinociceptive drugs of the gabapentinoid family has remained poorly understood. Gabapentin (GBP) binds to an exofacial epitope of the alpha(2)delta-1 and alpha(2)delta-2 auxiliary subunits of voltage-gated calcium channels, but acute inhibition of calcium currents by GBP is either very minor or absent. We formulated the hypothesis that GBP impairs the ability of alpha(2)delta subunits to enhance voltage-gated Ca(2+)channel plasma membrane density by means of an effect on trafficking. Our results conclusively demonstrate that GBP inhibits calcium currents, mimicking a lack of alpha(2)delta only when applied chronically, but not acutely, both in heterologous expression systems and in dorsal root-ganglion neurons. GBP acts primarily at an intracellular location, requiring uptake, because the effect of chronically applied GBP is blocked by an inhibitor of the system-L neutral amino acid transporters and enhanced by coexpression of a transporter. However, it is mediated by alpha(2)delta subunits, being prevented by mutations in either alpha(2)delta-1 or alpha(2)delta-2 that abolish GBP binding, and is not observed for alpha(2)delta-3, which does not bind GBP. Furthermore, the trafficking of alpha(2)delta-2 and Ca(V)2 channels is disrupted both by GBP and by the mutation in alpha(2)delta-2, which prevents GBP binding, and we find that GBP reduces cell-surface expression of alpha(2)delta-2 and Ca(V)2.1 subunits. Our evidence indicates that GBP may act chronically by displacing an endogenous ligand that is normally a positive modulator of alpha(2)delta subunit function, thereby impairing the trafficking function of the alpha(2)delta subunits to which it binds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
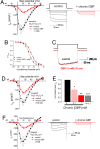
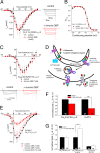
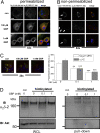

References
-
- Davies A, et al. Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends Pharmacol Sci. 2007;28:220–228. - PubMed
-
- Dolphin AC. β subunits of voltage-gated calcium channels. J Bioeng Biomemb. 2003;35:599–620. - PubMed
-
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. - PubMed
-
- Arikkath J, Campbell KP. Auxiliary subunits: Essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13:298–307. - PubMed
-
- Jay SD, et al. Structural characterization of the dihydropyridine-sensitive calcium channel α2-subunit and the associated δ peptides. J Biol Chem. 1991;266:3287–3293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

