Gene transfer in human vestibular epithelia and the prospects for inner ear gene therapy
- PMID: 18300702
- PMCID: PMC2642479
- DOI: 10.1097/MLG.0b013e318164d0aa
Gene transfer in human vestibular epithelia and the prospects for inner ear gene therapy
Abstract
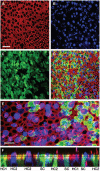
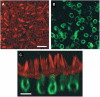
Transfer of exogenous genetic material into the mammalian inner ear using viral vectors has been characterized over the last decade. A number of different viral vectors have been shown to transfect the varying cell types of the nonprimate mammalian inner ear. Several routes of delivery have been identified for introduction of vectors into the inner ear while minimizing injury to existing structures and at the same time ensuring widespread distribution of the agent throughout the cochlea and the rest of the inner ear. These studies raise the possibility that gene transfer may be developed as a potential strategy for treating inner ear dysfunction in humans. Furthermore, a recent report showing successful transfection of excised human vestibular epithelia offers proof of principle that viral gene transfer is a viable strategy for introduction and expression of exogenous genetic material to restore function to the inner ear. Human vestibular epithelia were harvested from patients undergoing labyrinthectomy, either for intractable Ménière's disease or vestibular schwannoma resection, and cultured for as long as 5 days. In those experiments, recombinant, multiply-deleted, replication-deficient adenoviral vectors were used to transfect and express a reporter gene as well as the functionally relevant gene, wild-type KCNQ4, a potassium channel gene that when mutated causes the autosomal dominant HL DFNA2.Here, we review the current state of viral-mediated gene transfer in the inner ear and discuss different viral vectors, routes of delivery, and potential applications of gene therapy. Emphasis is placed on experiments demonstrating viral transfection of human inner ear tissue and implications of these findings and for the future of gene therapy in the human inner ear.
Figures

References
-
- Lalwani AK, Castelein CM. Cracking the auditory genetic code: nonsyndromic hereditary hearing impairment. Am J Otol. 1999;20:115–132. - PubMed
-
- Raphael Y, Frisancho JC, Roessler BJ. Adenoviral-mediated gene transfer into guinea pig cochlear cells in vivo. Neurosci Lett. 1996;207:137–141. - PubMed
-
- Weiss MA, Frisancho JC, Roessler BJ, Raphael Y. Viral-mediated gene transfer in the cochlea. Int J Devel Neurosci. 1997;15:577–583. - PubMed
-
- Dazert Transfection of neonatal rat cochlear cells in vitro with an adenovirus vector. Int J Devel Neurosci. 1997;15:595–600. - PubMed
-
- Lalwani AK, Walsh BJ, Reilly PG, Muzyczka N, Mhatre AN. Development of in vivo gene therapy for hearing disorders: introduction of adeno-associated virus into the cochlea of the guinea pig. Gene Ther. 1996;3:588–592. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

