Augmentation of neovascularization [corrected] in hindlimb ischemia by combined transplantation of human embryonic stem cells-derived endothelial and mural cells
- PMID: 18301744
- PMCID: PMC2244708
- DOI: 10.1371/journal.pone.0001666
Augmentation of neovascularization [corrected] in hindlimb ischemia by combined transplantation of human embryonic stem cells-derived endothelial and mural cells
Erratum in
- PLoS ONE. 2009;4(1). doi: 10.1371/annotation/7f0f9ad9-d300-4228-a7ec-65d906b08d2d doi: 10.1371/annotation/7f0f9ad9-d300-4228-a7ec-65d906b08d2d
Abstract
Background: We demonstrated that mouse embryonic stem (ES) cells-derived vascular endothelial growth factor receptor-2 (VEGF-R2) positive cells could differentiate into both endothelial cells (EC) and mural cells (MC), and termed them as vascular progenitor cells (VPC). Recently, we have established a method to expand monkey and human ES cells-derived VPC with the proper differentiation stage in a large quantity. Here we investigated the therapeutic potential of human VPC-derived EC and MC for vascular regeneration.
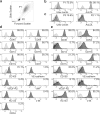
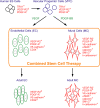
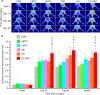
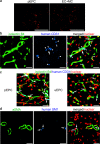
Methods and results: After the expansion of human VPC-derived vascular cells, we transplanted these cells to nude mice with hindlimb ischemia. The blood flow recovery and capillary density in ischemic hindlimbs were significantly improved in human VPC-derived EC-transplanted mice, compared to human peripheral and umbilical cord blood-derived endothelial progenitor cells (pEPC and uEPC) transplanted mice. The combined transplantation of human VPC-derived EC and MC synergistically improved blood flow of ischemic hindlimbs remarkably, compared to the single cell transplantations. Transplanted VPC-derived vascular cells were effectively incorporated into host circulating vessels as EC and MC to maintain long-term vascular integrity.
Conclusions: Our findings suggest that the combined transplantation of human ES cells-derived EC and MC can be used as a new promising strategy for therapeutic vascular regeneration in patients with tissue ischemia.
Conflict of interest statement
Figures


References
-
- Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. - PubMed
-
- Yurugi-Kobayashi T, Itoh H, Yamashita J, Yamahara K, Hirai H, et al. Effective contribution of transplanted vascular progenitor cells derived from embryonic stem cells to adult neovascularization in proper differentiation stage. Blood. 2003;101:2675–2678. - PubMed
-
- Sone M, Itoh H, Yamashita J, Yurugi-Kobayashi T, Suzuki Y, et al. Different differentiation kinetics of vascular progenitor cells in primate and mouse embryonic stem cells. Circulation. 2003;107:2085–2088. - PubMed
-
- Sone M, Itoh H, Yamahara K, Yamashita JK, Yurugi-Kobayashi T, et al. Pathway for differentiation of human embryonic stem cells to vascular cell components and their potential for vascular regeneration. Arterioscler Thromb Vasc Biol. 2007;27:2127–2134. - PubMed
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

