SOD1 and amyotrophic lateral sclerosis: mutations and oligomerization
- PMID: 18301754
- PMCID: PMC2250751
- DOI: 10.1371/journal.pone.0001677
SOD1 and amyotrophic lateral sclerosis: mutations and oligomerization
Abstract
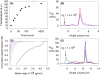
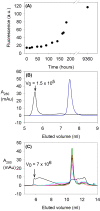
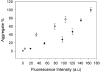
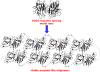
There are about 100 single point mutations of copper, zinc superoxide dismutase 1 (SOD1) which are reported (http://alsod.iop.kcl.ac.uk/Als/index.aspx) to be related to the familial form (fALS) of amyotrophic lateral sclerosis (ALS). These mutations are spread all over the protein. It is well documented that fALS produces protein aggregates in the motor neurons of fALS patients, which have been found to be associated to mitochondria. We selected eleven SOD1 mutants, most of them reported as pathological, and characterized them investigating their propensity to aggregation using different techniques, from circular dichroism spectra to ThT-binding fluorescence, size-exclusion chromatography and light scattering spectroscopy. We show here that these eleven SOD1 mutants, only when they are in the metal-free form, undergo the same general mechanism of oligomerization as found for the WT metal-free protein. The rates of oligomerization are different but eventually they give rise to the same type of soluble oligomeric species. These oligomers are formed through oxidation of the two free cysteines of SOD1 (6 and 111) and stabilized by hydrogen bonds, between beta strands, thus forming amyloid-like structures. SOD1 enters the mitochondria as demetallated and mitochondria are loci where oxidative stress may easily occur. The soluble oligomeric species, formed by the apo form of both WT SOD1 and its mutants through an oxidative process, might represent the precursor toxic species, whose existence would also suggest a common mechanism for ALS and fALS. The mechanism here proposed for SOD1 mutant oligomerization is absolutely general and it provides a common unique picture for the behaviors of the many SOD1 mutants, of different nature and distributed all over the protein.
Conflict of interest statement
Figures


References
-
- Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. - PubMed
-
- Rosen DR. Mutation in Cu,Zn superoxide dismutase gene are associated with familial amyotrophyc lateral sclerosis. Nature. 1993;362:59–62. - PubMed
-
- Valentine JS, Doucette PA, Potter SZ. Copper-Zinc Superoxide Dismutase and Amyotrophic Lateral Sclerosis. Annu Rev Biochem. 2005;74:563–593. - PubMed
-
- Andersen PM. Amyotrophic lateral sclerosis associated with mutations in the CuZn superoxide dismutase gene. Curr Neurol Neurosci Rep. 2006;6:37–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

