Synthetic biology of proteins: tuning GFPs folding and stability with fluoroproline
- PMID: 18301757
- PMCID: PMC2243022
- DOI: 10.1371/journal.pone.0001680
Synthetic biology of proteins: tuning GFPs folding and stability with fluoroproline
Abstract
Background: Proline residues affect protein folding and stability via cis/trans isomerization of peptide bonds and by the C(gamma)-exo or -endo puckering of their pyrrolidine rings. Peptide bond conformation as well as puckering propensity can be manipulated by proper choice of ring substituents, e.g. C(gamma)-fluorination. Synthetic chemistry has routinely exploited ring-substituted proline analogs in order to change, modulate or control folding and stability of peptides.
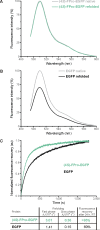
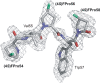
Methodology/principal findings: In order to transmit this synthetic strategy to complex proteins, the ten proline residues of enhanced green fluorescent protein (EGFP) were globally replaced by (4R)- and (4S)-fluoroprolines (FPro). By this approach, we expected to affect the cis/trans peptidyl-proline bond isomerization and pyrrolidine ring puckering, which are responsible for the slow folding of this protein. Expression of both protein variants occurred at levels comparable to the parent protein, but the (4R)-FPro-EGFP resulted in irreversibly unfolded inclusion bodies, whereas the (4S)-FPro-EGFP led to a soluble fluorescent protein. Upon thermal denaturation, refolding of this variant occurs at significantly higher rates than the parent EGFP. Comparative inspection of the X-ray structures of EGFP and (4S)-FPro-EGFP allowed to correlate the significantly improved refolding with the C(gamma)-endo puckering of the pyrrolidine rings, which is favored by 4S-fluorination, and to lesser extents with the cis/trans isomerization of the prolines.
Conclusions/significance: We discovered that the folding rates and stability of GFP are affected to a lesser extent by cis/trans isomerization of the proline bonds than by the puckering of pyrrolidine rings. In the C(gamma)-endo conformation the fluorine atoms are positioned in the structural context of the GFP such that a network of favorable local interactions is established. From these results the combined use of synthetic amino acids along with detailed structural knowledge and existing protein engineering methods can be envisioned as a promising strategy for the design of complex tailor-made proteins and even cellular structures of superior properties compared to the native forms.
Conflict of interest statement
Figures

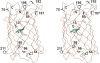

Similar articles
-
Rational design of protein stability: effect of (2S,4R)-4-fluoroproline on the stability and folding pathway of ubiquitin.PLoS One. 2011;6(5):e19425. doi: 10.1371/journal.pone.0019425. Epub 2011 May 16. PLoS One. 2011. PMID: 21625626 Free PMC article.
-
Modulating the folding stability and ligand binding affinity of Pin1 WW domain by proline ring puckering.Proteins. 2014 Jan;82(1):67-76. doi: 10.1002/prot.24359. Epub 2013 Aug 31. Proteins. 2014. PMID: 23839950
-
4,4-Difluoroproline as a Unique 19F NMR Probe of Proline Conformation.Biochemistry. 2024 May 7;63(9):1131-1146. doi: 10.1021/acs.biochem.3c00697. Epub 2024 Apr 10. Biochemistry. 2024. PMID: 38598681
-
Biochemistry of fluoroprolines: the prospect of making fluorine a bioelement.Beilstein J Org Chem. 2021 Feb 15;17:439-460. doi: 10.3762/bjoc.17.40. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 33727970 Free PMC article. Review.
-
Proline Analogues.Chem Rev. 2024 Jul 10;124(13):8130-8232. doi: 10.1021/acs.chemrev.4c00007. Epub 2024 Jun 28. Chem Rev. 2024. PMID: 38941181 Review.
Cited by
-
Polarity effects in 4-fluoro- and 4-(trifluoromethyl)prolines.Beilstein J Org Chem. 2020 Jul 23;16:1837-1852. doi: 10.3762/bjoc.16.151. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 32765799 Free PMC article.
-
Incorporation of proline analogs into recombinant proteins expressed in Escherichia coli.Methods Enzymol. 2021;656:545-571. doi: 10.1016/bs.mie.2021.05.008. Epub 2021 Jun 18. Methods Enzymol. 2021. PMID: 34325798 Free PMC article.
-
Protein evolution via amino acid and codon elimination.PLoS One. 2010 Apr 26;5(4):e10104. doi: 10.1371/journal.pone.0010104. PLoS One. 2010. PMID: 20436666 Free PMC article.
-
Designing protein-based biomaterials for medical applications.Acta Biomater. 2014 Apr;10(4):1542-57. doi: 10.1016/j.actbio.2013.10.001. Epub 2013 Oct 9. Acta Biomater. 2014. PMID: 24121196 Free PMC article. Review.
-
Residue-specific incorporation of non-canonical amino acids into proteins: recent developments and applications.Curr Opin Chem Biol. 2010 Dec;14(6):774-80. doi: 10.1016/j.cbpa.2010.09.013. Epub 2010 Nov 9. Curr Opin Chem Biol. 2010. PMID: 21071259 Free PMC article. Review.
References
-
- Palm GJ, Wlodawer A. Spectral variants of green fluorescent protein. Methods Enzymol. 1999;302:378–394. - PubMed
-
- Battistutta R, Negro A, Zanotti G. Crystal structure and refolding properties of the mutant F99S/M153T/V163A of the green fluorescent protein. Proteins. 2000;41:429–437. - PubMed
-
- Reid BG, Flynn GC. Chromophore Formation in Green Fluorescent Protein. Biochemistry. 1997;36:6786–6791. - PubMed
-
- Fischer G, Aumüller T. Reviews of Physiology, Biochemistry and Pharmacology. Berlin: Springer Verlag; 2004. Regulation of peptide bond cis/trans isomerization by enzyme catalysis and its implication in physiological processes. pp. 105–150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

