IdeS: a bacterial proteolytic enzyme with therapeutic potential
- PMID: 18301769
- PMCID: PMC2253494
- DOI: 10.1371/journal.pone.0001692
IdeS: a bacterial proteolytic enzyme with therapeutic potential
Abstract
Background: IdeS, a proteinase from Streptococcus pyogenes, cleaves immunoglobulin (Ig)G antibodies with a unique degree of specificity. Pathogenic IgG antibodies constitute an important clinical problem contributing to the pathogenesis of a number of autoimmune conditions and acute transplant rejection. To be able to effectively remove such antibodies is therefore an important clinical challenge.
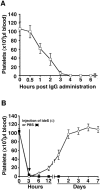
Methodology/principal findings: IdeS was found to specifically and efficiently cleave IgG in human blood in vitro (20 microg of IdeS caused a complete degradation of IgG in one ml of human whole blood in 15 minutes) and to clear IgG from the blood stream of rabbits in vivo (no IgG was detected six hours following an intravenous injection of 5 mg of IdeS) without any side effects. In a mouse model of immune thrombocytopenic purpura (ITP), polyclonal IgG antibodies against platelet surface antigens were used to induce a lethal disease. These profoundly thrombocytopenic animals were treated and cured by a single injection of IdeS.
Conclusions/significance: Novel information is provided concerning the IgG-cleaving activity of IdeS in vitro and in vivo. The highly specific and rapid elimination of IgG in vivo, the dramatic effect in a mouse model of ITP, and the lack of side effects in the treated animals, indicate that IdeS could also be used to treat IgG-driven diseases in humans.
Conflict of interest statement
Figures
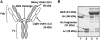
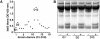
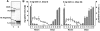

References
-
- NIH Autoimmune Disease Coordinating Committee Report. 2002. In Bethseda, MD.
-
- Bygren P, Freiburghaus C, Lindholm T, Simonsen O, Thysell H, et al. Goodpasture's syndrome treated with staphylococcal protein A immunoadsorption. Lancet. 1985;2:1295–1296. - PubMed
-
- Palmer A, Gjorstrup P, Severn A, Welsh K, Taube D. Treatment of systemic lupus erythematosus by extracorporeal immunoadsorption. Lancet. 1988;2:272. - PubMed
-
- Palmer A, Taube D, Welsh K, Bewick M, Gjorstrup P, et al. Removal of anti-HLA antibodies by extracorporeal immunoadsorption to enable renal transplantation. Lancet. 1989;1:10–12. - PubMed
-
- Berta E, Confalonieri P, Simoncini O, Bernardi G, Busnach G, et al. Removal of antiacetylcholine receptor antibodies by protein-A immunoadsorption in myasthenia gravis. Int J Artif Organs. 1994;17:603–608. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

