Synthesis of dihydropyridines and pyridines from imines and alkynes via C-H activation
- PMID: 18302381
- PMCID: PMC3057408
- DOI: 10.1021/ja7104784
Synthesis of dihydropyridines and pyridines from imines and alkynes via C-H activation
Abstract
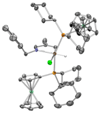
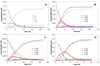
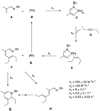
A convenient one-pot C-H alkenylation/electrocyclization/aromatization sequence has been developed for the synthesis of highly substituted pyridine derivatives from alkynes and alpha,beta-unsaturated N-benzyl aldimines and ketimines that proceeds through dihydropyridine intermediates. A new class of ligands for C-H activation was developed, providing broader scope for the alkenylation step than could be achieved with previously reported ligands. Substantial information was obtained about the mechanism of the reaction. This included the isolation of a C-H activated complex and its structure determination by X-ray analysis; in addition, kinetic simulations using the Copasi software were employed to determine rate constants for this transformation, implicating facile C-H oxidative addition and slow reductive elimination steps.
Figures


References
-
- Joule JA, Mills K. Heterocyclic Chemistry. 4th ed. Oxford, UK: Blackwell; 2000.
-
- Carey JS, Laffan D, Thomson C, Williams MT. Org. Biomol. Chem. 2006;4:2337–2347. - PubMed
-
-
For recent reviews on pyridine syntheses see the following and references therein: Varela J, Saa C. Chem. Rev. 2003;103:3787–3801. Zeni G, Larock RL. Chem. Rev. 2006;106:4644–4680. For recent examples of transition-metal facilitated pyridine syntheses see: Trost BM, Gutierrez AC. Org. Lett. 2007;9:1473–1476. Movassaghi M, Hill MD. J. Am. Chem. Soc. 2006;128:4592–4593. McCormick MM, Duong HA, Zuo G, Louie J. J. Am. Chem. Soc. 2005;127:5030–5031. Yamamoto Y, Kinpara K, Ogawa R, Nishiyama H, Itoh K. Chem. Eur. J. 2006;12:5618–5631. Tanaka R, Yuza A, Watai Y, Suzuki D, Takayama Y, Sato F, Urabe H. J. Am. Chem. Soc. 2005;127:7774–7780. Takahashi T, Tsai F-Y, Li Y, Wang H, Kondo Y, Yamanaka M, Nakajima K, Kotora M. J. Am. Chem. Soc. 2002;124:5059–5067. For pyridine syntheses from Diels Alder inputs see: Boger DL. Chem. Rev. 1986;86:781–793. Fletchter MD, Hurst TE, Miles TJ, Moody CJ. Tetrahedron. 2006;62:5454–5463..
-
-
- Movassaghi M, Hill MD, Ahmad OK. J. Am. Chem. Soc. 2007;129:10096–10097. - PubMed
-
-
For an example of pyridine synthesis from oxime and alkyne inputs via C-H activation see: Parthasarathy K, Jeganmohan M, Cheng C-H. Org. Lett. 2007 ASAP.
-

