Effect of ionic strength and presence of serum on lipoplexes structure monitorized by FRET
- PMID: 18302788
- PMCID: PMC2275333
- DOI: 10.1186/1472-6750-8-20
Effect of ionic strength and presence of serum on lipoplexes structure monitorized by FRET
Abstract
Background: Serum and high ionic strength solutions constitute important barriers to cationic lipid-mediated intravenous gene transfer. Preparation or incubation of lipoplexes in these media results in alteration of their biophysical properties, generally leading to a decrease in transfection efficiency. Accurate quantification of these changes is of paramount importance for the success of lipoplex-mediated gene transfer in vivo.
Results: In this work, a novel time-resolved fluorescence resonance energy transfer (FRET) methodology was used to monitor lipoplex structural changes in the presence of phosphate-buffered saline solution (PBS) and fetal bovine serum. 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP)/pDNA lipoplexes, prepared in high and low ionic strength solutions, are compared in terms of complexation efficiency. Lipoplexes prepared in PBS show lower complexation efficiencies when compared to lipoplexes prepared in low ionic strength buffer followed by addition of PBS. Moreover, when serum is added to the referred formulation no significant effect on the complexation efficiency was observed. In physiological saline solutions and serum, a multilamellar arrangement of the lipoplexes is maintained, with reduced spacing distances between the FRET probes, relative to those in low ionic strength medium.
Conclusion: The time-resolved FRET methodology described in this work allowed us to monitor stability and characterize quantitatively the structural changes (variations in interchromophore spacing distances and complexation efficiencies) undergone by DOTAP/DNA complexes in high ionic strength solutions and in presence of serum, as well as to determine the minimum amount of potentially cytotoxic cationic lipid necessary for complete coverage of DNA. This constitutes essential information regarding thoughtful design of future in vivo applications.
Figures


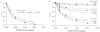
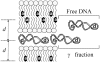
Similar articles
-
Liposome complexation efficiency monitored by FRET: effect of charge ratio, helper lipid and plasmid size.Eur Biophys J. 2007 Jul;36(6):609-20. doi: 10.1007/s00249-007-0130-x. Epub 2007 Jan 30. Eur Biophys J. 2007. PMID: 17262222
-
Characterization and in vivo performance of dextran-spermine polyplexes and DOTAP/cholesterol lipoplexes administered locally and systemically.Biomaterials. 2007 May;28(14):2339-49. doi: 10.1016/j.biomaterials.2006.09.001. Epub 2007 Feb 12. Biomaterials. 2007. PMID: 17298842
-
Factors affecting DNA binding and stability of association to cationic liposomes.Chem Phys Lipids. 2012 May;165(4):414-23. doi: 10.1016/j.chemphyslip.2012.03.006. Chem Phys Lipids. 2012. PMID: 22715503
-
Transferrin-associated lipoplexes as gene delivery systems: relevance of mode of preparation and biophysical properties.J Membr Biol. 2008 Feb;221(3):141-52. doi: 10.1007/s00232-008-9092-x. Epub 2008 Feb 21. J Membr Biol. 2008. PMID: 18288435
-
DOTAP (and other cationic lipids): chemistry, biophysics, and transfection.Crit Rev Ther Drug Carrier Syst. 2004;21(4):257-317. doi: 10.1615/critrevtherdrugcarriersyst.v21.i4.10. Crit Rev Ther Drug Carrier Syst. 2004. PMID: 15638468 Review.
Cited by
-
Influence of biological media on the structure and behavior of ferrocene-containing cationic lipid/DNA complexes used for DNA delivery.Langmuir. 2011 Jun 7;27(11):6615-21. doi: 10.1021/la200450x. Epub 2011 May 2. Langmuir. 2011. PMID: 21528933 Free PMC article.
-
Enhancing the In Vitro and In Vivo Stabilities of Polymeric Nucleic Acid Delivery Nanosystems.Bioconjug Chem. 2019 Feb 20;30(2):325-337. doi: 10.1021/acs.bioconjchem.8b00749. Epub 2018 Dec 28. Bioconjug Chem. 2019. PMID: 30592619 Free PMC article. Review.
-
Multi-Step Assembly of an RNA-Liposome Nanoparticle Formulation Revealed by Real-Time, Single-Particle Quantitative Imaging.Adv Sci (Weinh). 2025 Mar;12(12):e2414305. doi: 10.1002/advs.202414305. Epub 2025 Jan 31. Adv Sci (Weinh). 2025. PMID: 39887619 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

