Getting in and out from calnexin/calreticulin cycles
- PMID: 18303019
- PMCID: PMC2447651
- DOI: 10.1074/jbc.R700048200
Getting in and out from calnexin/calreticulin cycles
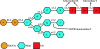
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

