Targeted gene knock in and sequence modulation mediated by a psoralen-linked triplex-forming oligonucleotide
- PMID: 18303025
- PMCID: PMC2431054
- DOI: 10.1074/jbc.M800607200
Targeted gene knock in and sequence modulation mediated by a psoralen-linked triplex-forming oligonucleotide
Abstract
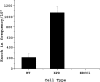
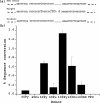
Information from exogenous donor DNA can be introduced into the genome via homology-directed repair (HDR) pathways. These pathways are stimulated by double strand breaks and by DNA damage such as interstrand cross-links. We have employed triple helix-forming oligonucleotides linked to psoralen (pso-TFO) to introduce a DNA interstrand cross-link at a specific site in the genome of living mammalian cells. Co-introduction of duplex DNA with target region homology resulted in precise knock in of the donor at frequencies 2-3 orders of magnitude greater than with donor alone. Knock-in was eliminated in cells deficient in ERCC1-XPF, which is involved in recombinational pathways as well as cross-link repair. Separately, single strand oligonucleotide donors (SSO) were co-introduced with the pso-TFO. These were 10-fold more active than the duplex knock-in donor. SSO efficacy was further elevated in cells deficient in ERCC1-XPF, in contrast to the duplex donor. Resected single strand ends have been implicated as critical intermediates in sequence modulation by SSO, as well as duplex donor knock in. We asked whether there would be a competition between the donor species for these ends if both were present with the pso-TFO. The frequency of duplex donor knock in was unaffected by a 100-fold molar excess of the SSO. The same result was obtained when the homing endonuclease I-SceI was used to initiate HDR at the target site. We conclude that the entry of double strand breaks into distinct HDR pathways is controlled by factors other than the nucleic acid partners in those pathways.
Figures






Similar articles
-
Repair of DNA lesions associated with triplex-forming oligonucleotides.Mol Carcinog. 2009 Apr;48(4):389-99. doi: 10.1002/mc.20501. Mol Carcinog. 2009. PMID: 19072762 Free PMC article. Review.
-
Triplex targeted genomic crosslinks enter separable deletion and base substitution pathways.Nucleic Acids Res. 2005 Sep 25;33(17):5382-93. doi: 10.1093/nar/gki851. Print 2005. Nucleic Acids Res. 2005. PMID: 16186129 Free PMC article.
-
Processing of a psoralen DNA interstrand cross-link by XPF-ERCC1 complex in vitro.J Biol Chem. 2008 Jan 18;283(3):1275-1281. doi: 10.1074/jbc.M708072200. Epub 2007 Nov 15. J Biol Chem. 2008. PMID: 18006494
-
Repair of an interstrand DNA cross-link initiated by ERCC1-XPF repair/recombination nuclease.J Biol Chem. 2000 Aug 25;275(34):26632-6. doi: 10.1074/jbc.C000337200. J Biol Chem. 2000. PMID: 10882712
-
Targeted genome modification via triple helix formation.Ann N Y Acad Sci. 2005 Nov;1058:151-61. doi: 10.1196/annals.1359.023. Ann N Y Acad Sci. 2005. PMID: 16394134 Review.
Cited by
-
Triplex-forming oligonucleotides as an anti-gene technique for cancer therapy.Front Pharmacol. 2022 Dec 21;13:1007723. doi: 10.3389/fphar.2022.1007723. eCollection 2022. Front Pharmacol. 2022. PMID: 36618947 Free PMC article. Review.
-
Advances in understanding the complex mechanisms of DNA interstrand cross-link repair.Cold Spring Harb Perspect Biol. 2013 Oct 1;5(10):a012732. doi: 10.1101/cshperspect.a012732. Cold Spring Harb Perspect Biol. 2013. PMID: 24086043 Free PMC article. Review.
-
Development and functional evaluation of a psoralen-conjugated nucleoside mimic for triplex-forming oligonucleotides.Commun Chem. 2025 Jan 22;8(1):18. doi: 10.1038/s42004-025-01416-2. Commun Chem. 2025. PMID: 39843926 Free PMC article.
-
Repair of DNA lesions associated with triplex-forming oligonucleotides.Mol Carcinog. 2009 Apr;48(4):389-99. doi: 10.1002/mc.20501. Mol Carcinog. 2009. PMID: 19072762 Free PMC article. Review.
-
An update on targeted gene repair in mammalian cells: methods and mechanisms.J Biomed Sci. 2011 Feb 2;18(1):10. doi: 10.1186/1423-0127-18-10. J Biomed Sci. 2011. PMID: 21284895 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

