Economic and clinical benefits of galantamine in the treatment of mild to moderate Alzheimer's disease in a Korean population: a 52-week prospective study
- PMID: 18303192
- PMCID: PMC2526501
- DOI: 10.3346/jkms.2008.23.1.10
Economic and clinical benefits of galantamine in the treatment of mild to moderate Alzheimer's disease in a Korean population: a 52-week prospective study
Abstract
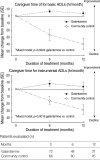
To evaluate the impact of galantamine treatment on the function, caregiver time, and resource used in the treatment of patients with mild to moderate Alzheimer's disease (AD), costs and outcomes were evaluated during a 52-week prospective, randomized, double-blind, community-controlled trial of galantamine. Patients received either galantamine treatment (n=72) or no treatment (n=66). The analysis was performed from a societal perspective. Galantamine treatment reduced time spent caring for the patients and maintained improved functional capacity, whereas, when no treatments were given, a great increase in caregiver time and progressive functional deteriorations were observed. Saved caregiver time was equivalent to 113 working days per year. After 52 weeks, mean total annual costs per patient were 14,735,000 Korea Won (KRW) (USD 12,315) for patients with galantamine treatment and 25,325,000 KRW (USD 21,166) for patients without treatment. Adjusted annual cost saving of galantamine treatment was 6,428,000 KRW (USD 5,372) when compared to no treatment (p=0.0089). Galantamine had a beneficial effect not only to slow functional decline in patients with mild to moderate AD, but also to save a substantial amount of costs, closely related to reduction in caregiver burden and decrease in caregiver time.
Figures
References
-
- Suh GH, Kim JK, Cho MJ. Community study of dementia in the older Korean population. Aust N Z J Psychiatry. 2003;37:606–612. - PubMed
-
- Bores GM, Huger FP, Petko W, Mutlib AE, Camacho F, Rush DK, Selk DE, Wof V, Kosley RW Jr, Davis L, Vargas HM. Pharmacological evaluation of novel Alzheimer's disease therapeutics: acetylcholinesterase inhibitors related to galanthamine. J Pharmacol Exp Ther. 1996;277:728–738. - PubMed
-
- Chu LW, Yik PY, Mok W, Chung CP. A 2-year open-label study of galantamine therapy in Chinese Alzheimer's disease patients in Hong Kong. Int J Clin Pract. 2007;61:403–410. - PubMed
-
- Pirttila T, Wilcock G, Truyen L, Damaraju CV. Long-term efficacy and safety of galantamine in patients with mild to moderate Alzheimer's disease: multicentre trial. Eur J Neurol. 2004;11:734–741. - PubMed
-
- Suh GH, Jung HY, Lee CU, Oh BH, Bae JN, Jung HY, Ju YS, Yeon BK, Park J, Hong I, Choi S, Lee HJ. A prospective, double-blind, community-controlled comparison of three doses of galantamine in the treatment of mild to moderate Alzheimer's disease in a Korean population. Clin Ther. 2004;26:1608–1618. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical