Asynchronous decoding of dexterous finger movements using M1 neurons
- PMID: 18303800
- PMCID: PMC2780566
- DOI: 10.1109/TNSRE.2007.916289
Asynchronous decoding of dexterous finger movements using M1 neurons
Erratum in
- IEEE Trans Neural Syst Rehabil Eng. 2008 Aug;16(4):421
Abstract
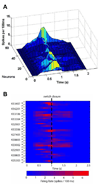
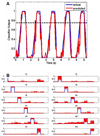
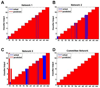
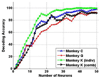
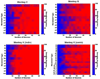
Previous efforts in brain-machine interfaces (BMI) have looked at decoding movement intent or hand and arm trajectory, but current cortical control strategies have not focused on the decoding of dexterous [corrected] actions such as finger movements. The present work demonstrates the asynchronous decoding (i.e., where cues indicating the onset of movement are not known) of individual and combined finger movements. Single-unit activities were recorded sequentially from a population of neurons in the M1 hand area of trained rhesus monkeys during flexion and extension movements of each finger and the wrist. Nonlinear filters were designed to detect the onset of movement and decode the movement type from randomly selected neuronal ensembles (assembled from individually recorded single-unit activities). Average asynchronous decoding accuracies as high as 99.8%, 96.2%, and 90.5%, were achieved for individuated finger and wrist movements with three monkeys. Average decoding accuracy was still 92.5% when combined movements of two fingers were included. These results demonstrate that it is possible to asynchronously decode dexterous finger movements from a neuronal ensemble with high accuracy. This work takes an important step towards the development of a BMI for direct neural control of a state-of-the-art, multifingered hand prosthesis.
Figures
References
-
- Santhanam G, Ryu SI, Yu BM, Afshar A, Shenoy KV. A high-performance brain-computer interface. Nature. 2006 Jul 13;vol. 442:195–198. - PubMed
-
- Wessberg J, Stambaugh CR, Kralik JD, Beck PD, Laubach M, Chapin JK, Kim J, Biggs SJ, Srinivasan MA, Nicolelis MA. Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature. 2000 Nov 16;vol. 408:361–365. - PubMed
-
- Chapin JK, Moxon KA, Markowitz RS, Nicolelis MA. Real-time control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat Neurosci. 1999 Jul;vol. 2:664–670. - PubMed
-
- Fetz EE. Real-time control of a robotic arm by neuronal ensembles. Nat Neurosci. 1999 Jul;vol. 2:583–584. - PubMed
-
- Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006 Jul 13;vol. 442:164–171. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

