Total synthesis of the strychnos alkaloid (+)-minfiensine: tandem enantioselective intramolecular Heck-iminium ion cyclization
- PMID: 18303837
- PMCID: PMC2995331
- DOI: 10.1021/ja800163v
Total synthesis of the strychnos alkaloid (+)-minfiensine: tandem enantioselective intramolecular Heck-iminium ion cyclization
Abstract
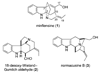
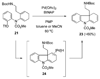
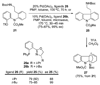
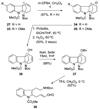
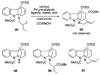
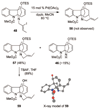
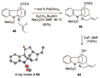
A 1,2,3,4-tetrahydro-9a,4a-(iminoethano)-9H-carbazole (4) is a central structural feature of the Strychnos alkaloid minfiensine (1) and akuammiline alkaloids such as vincorine (5) and echitamine (6). A cascade catalytic asymmetric Heck-iminium cyclization was developed that rapidly provides 3,4-dihydro-9a,4a-(iminoethano)-9H-carbazoles in high enantiomeric purity. Two sequences were developed for advancing 3,4-dihydro-9a,4a-(iminoethano)-9H-carbazole 27 to (+)-minfiensine. In our first-generation approach, a reductive Heck cyclization was employed to form the fifth ring of (+)-minfiensine. In a second more concise total synthesis, an intramolecular palladium-catalyzed ketone enolate vinyl iodide coupling was employed to construct the final ring of (+)-minfiensine. This second-generation total synthesis of enantiopure (+)-minfiensine was accomplished in 6.5% overall yield and 15 steps from 1,2-cyclohexanedione and anisidine 13. A distinctive feature of this sequence is the use of palladium-catalyzed reactions to form all carbon-carbon bonds in the transformation of these simple precursors to (+)-minfiensine.
Figures













References
-
- Massiot G, Thépenier P, Jacquier M-J, Le Men-Olivier L, Delaude C. Heterocycles. 1989;29:1435–1438.
-
-
For review, see: Higuchi K, Kawasaki T. Nat. Prod. Rep. 2007;24:843–868., and earlier articles in this series.
-
-
- Anthoni U, Christophersen C, Nielsen PH. In: Alkaloids: Chemical and Biological Perspectives. Pelletier SW, editor. Vol. 13. New York: Wiley; 1999. pp. 163–236.
-
- Ramirez A, Garcia-Rubio S. Curr. Med. Chem. 2003;10:1891–1915. - PubMed
-
- Dounay AB, Overman LE, Wrobleski AD. J. Am. Chem. Soc. 2005;127:10186–10187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

