Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration
- PMID: 18303940
- PMCID: PMC2253608
- DOI: 10.1371/journal.pmed.0050045
Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration
Abstract
Background: Meta-analyses of antidepressant medications have reported only modest benefits over placebo treatment, and when unpublished trial data are included, the benefit falls below accepted criteria for clinical significance. Yet, the efficacy of the antidepressants may also depend on the severity of initial depression scores. The purpose of this analysis is to establish the relation of baseline severity and antidepressant efficacy using a relevant dataset of published and unpublished clinical trials.
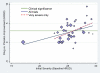
Methods and findings: We obtained data on all clinical trials submitted to the US Food and Drug Administration (FDA) for the licensing of the four new-generation antidepressants for which full datasets were available. We then used meta-analytic techniques to assess linear and quadratic effects of initial severity on improvement scores for drug and placebo groups and on drug-placebo difference scores. Drug-placebo differences increased as a function of initial severity, rising from virtually no difference at moderate levels of initial depression to a relatively small difference for patients with very severe depression, reaching conventional criteria for clinical significance only for patients at the upper end of the very severely depressed category. Meta-regression analyses indicated that the relation of baseline severity and improvement was curvilinear in drug groups and showed a strong, negative linear component in placebo groups.
Conclusions: Drug-placebo differences in antidepressant efficacy increase as a function of baseline severity, but are relatively small even for severely depressed patients. The relationship between initial severity and antidepressant efficacy is attributable to decreased responsiveness to placebo among very severely depressed patients, rather than to increased responsiveness to medication.
Conflict of interest statement
Figures


Comment in
-
Why do antidepressant therapies have such a poor success rate?Expert Rev Neurother. 2016 Jun;16(6):597-9. doi: 10.1586/14737175.2016.1158647. Epub 2016 Mar 28. Expert Rev Neurother. 2016. PMID: 26914370 No abstract available.
References
-
- National Institute for Clinical Excellence. Depression: management of depression in primary and secondary care. Clinical practice guideline No 23. London: National Institute for Clinical Excellence; 2004. 670
-
- Kirsch I, Moore TJ, Scoboria A, Nicholls SS. The emperor's new drugs: an analysis of antidepressant medication data submitted to the U.S. Food and Drug Administration. Prev Treat. 2002;5 article 23. Available: http://www.journals.apa.org/prevention/volume5/pre0050023a.html. Accessed 15 July 2002.
-
- Angst J. Severity of depression and benzodiazepine co-medication in relationship to efficacy of antidepressants in acute trials: a meta-analysis of moclobemide trials. Hum Psychopharmacol. 1993;8:401–407.
-
- Khan A, Leventhal R, Khan S, et al. Severity of depression and response to antidepressants and placebo: an analysis of the Food and Drug Administration database. J Clin Psychopharmacol. 2002;22:40–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

