Improvement in survival after paraquat ingestion following introduction of a new formulation in Sri Lanka
- PMID: 18303942
- PMCID: PMC2253611
- DOI: 10.1371/journal.pmed.0050049
Improvement in survival after paraquat ingestion following introduction of a new formulation in Sri Lanka
Abstract
Background: Pesticide ingestion is a common method of self-harm in the rural developing world. In an attempt to reduce the high case fatality seen with the herbicide paraquat, a novel formulation (INTEON) has been developed containing an increased emetic concentration, a purgative, and an alginate that forms a gel under the acid conditions of the stomach, potentially slowing the absorption of paraquat and giving the emetic more time to be effective. We compared the outcome of paraquat self-poisoning with the standard formulation against the new INTEON formulation following its introduction into Sri Lanka.
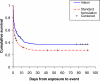
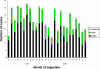
Methods and findings: Clinical data were prospectively collected on 586 patients with paraquat ingestion presenting to nine large hospitals across Sri Lanka with survival to 3 mo as the primary outcome. The identity of the formulation ingested after October 2004 was confirmed by assay of blood or urine samples for a marker compound present in INTEON. The proportion of known survivors increased from 76/297 with the standard formulation to 103/289 with INTEON ingestion, and estimated 3-mo survival improved from 27.1% to 36.7% (difference 9.5%; 95% confidence interval [CI] 2.0%-17.1%; p = 0.002, log rank test). Cox proportional hazards regression analyses showed an approximately 2-fold reduction in toxicity for INTEON compared to standard formulation. A higher proportion of patients ingesting INTEON vomited within 15 min (38% with the original formulation to 55% with INTEON, p < 0.001). Median survival time increased from 2.3 d (95% CI 1.2-3.4 d) with the standard formulation to 6.9 d (95% CI 3.3-10.7 d) with INTEON ingestion (p = 0.002, log rank test); however, in patients who did not survive there was a comparatively smaller increase in median time to death from 0.9 d (interquartile range [IQR] 0.5-3.4) to 1.5 d (IQR 0.5-5.5); p = 0.02.
Conclusions: The survey has shown that INTEON technology significantly reduces the mortality of patients following paraquat ingestion and increases survival time, most likely by reducing absorption.
Conflict of interest statement
Figures
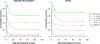
Comment in
-
New formulation of paraquat: a step forward but in the wrong direction.PLoS Med. 2008 Feb;5(2):e58. doi: 10.1371/journal.pmed.0050058. PLoS Med. 2008. PMID: 18303945 Free PMC article. Review.
References
-
- Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. Q J Med. 2000;93:715–731. - PubMed
-
- IPCS International Programme on Chemical Safety. Environmental Health Criteria No 39, Paraquat and Diquat. Geneva: World Health Organisation; 1984. 181
-
- Bismuth C, Hall AH, editors. Paraquat poisoning: mechanisms, prevention, treatment. New York: Marcel Dekker; 1995. 362
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

