Neurometabolism in human epilepsy
- PMID: 18304254
- PMCID: PMC3657747
- DOI: 10.1111/j.1528-1167.2008.01508.x
Neurometabolism in human epilepsy
Abstract
Purpose: Because of the large and continuous energetic requirements of brain function, neurometabolic dysfunction is a key pathophysiologic aspect of the epileptic brain. Additionally, neurometabolic dysfunction has many self-propagating features that are typical of epileptogenic processes, that is, where each occurrence makes the likelihood of further mitochondrial and energetic injury more probable. Thus abnormal neurometabolism may be not only a chronic accompaniment of the epileptic brain, but also a direct contributor to epileptogenesis.
Methods: We examine the evidence for neurometabolic dysfunction in epilepsy, integrating human studies of metabolic imaging, electrophysiology, microdialysis, as well as intracranial EEG and neuropathology.
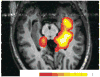
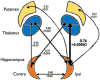
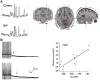
Results: As an approach of noninvasive functional imaging, quantitative magnetic resonance spectroscopic imaging (MRSI) measured abnormalities of mitochondrial and energetic dysfunction (via 1H or 31P spectroscopy) are related to several pathophysiologic indices of epileptic dysfunction. With patients undergoing hippocampal resection, intraoperative 13C-glucose turnover studies show a profound decrease in neurotransmitter (glutamate-glutamine) cycling relative to oxidation in the sclerotic hippocampus. Increased extracellular glutamate (which has long been associated with increased seizure likelihood) is significantly linked with declining energetics as measured by 31P MR, as well as with increased EEG measures of Teager energy, further arguing for a direct role of glutamate with hyperexcitability.
Discussion: Given the important contribution that metabolic performance makes toward excitability in brain, it is not surprising that numerous aspects of mitochondrial and energetic state link significantly with electrophysiologic and microdialysis measures in human epilepsy. This may be of particular relevance with the self-propagating nature of mitochondrial injury, but may also help define the conditions for which interventions may be developed.
Conflict of interest statement
Conflict of interest: The authors have declared no conflicts of interest.
Figures




References
-
- Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nat Rev Neurosci. 2005;6:829–840. - PubMed
-
- Araújo D, Santos AC, Velasco TR, Wichert-Ana L, Terra-Bustamante VC, Alexandre V, Carlotti CG, Assirati JA, Machado HR, Walz R, Leite JP, Sakamoto AC. Volumetric evidence of bilateral damage in unilateral mesial temporal lobe epilepsy. Epilepsia. 2006;47:1354–1359. - PubMed
-
- Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB. Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. Neuroreport. 1996;7:1397–1400. - PubMed
-
- Brines ML, Tabuteau H, Sundaresan S, Kim J, Spencer DD, de Lanerolle N. Regional distributions of hippocampal Na+,K(+)-ATPase, cytochrome oxidase, and total protein in temporal lobe epilepsy. Epilepsia. 1995;36:371–383. - PubMed
-
- Bruce AJ, Baudry M. Oxygen free radicals in rat limbic structures after kainate-induced seizures. Free Radic Biol Med. 1995;18:993–1002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

