Switching the mode of sucrose utilization by Saccharomyces cerevisiae
- PMID: 18304329
- PMCID: PMC2268662
- DOI: 10.1186/1475-2859-7-4
Switching the mode of sucrose utilization by Saccharomyces cerevisiae
Abstract
Background: Overflow metabolism is an undesirable characteristic of aerobic cultures of Saccharomyces cerevisiae during biomass-directed processes. It results from elevated sugar consumption rates that cause a high substrate conversion to ethanol and other bi-products, severely affecting cell physiology, bioprocess performance, and biomass yields. Fed-batch culture, where sucrose consumption rates are controlled by the external addition of sugar aiming at its low concentrations in the fermentor, is the classical bioprocessing alternative to prevent sugar fermentation by yeasts. However, fed-batch fermentations present drawbacks that could be overcome by simpler batch cultures at relatively high (e.g. 20 g/L) initial sugar concentrations. In this study, a S. cerevisiae strain lacking invertase activity was engineered to transport sucrose into the cells through a low-affinity and low-capacity sucrose-H+ symport activity, and the growth kinetics and biomass yields on sucrose analyzed using simple batch cultures.
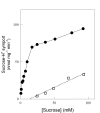
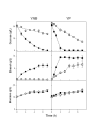
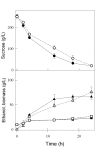
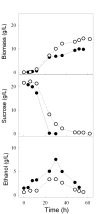
Results: We have deleted from the genome of a S. cerevisiae strain lacking invertase the high-affinity sucrose-H+ symporter encoded by the AGT1 gene. This strain could still grow efficiently on sucrose due to a low-affinity and low-capacity sucrose-H+ symport activity mediated by the MALx1 maltose permeases, and its further intracellular hydrolysis by cytoplasmic maltases. Although sucrose consumption by this engineered yeast strain was slower than with the parental yeast strain, the cells grew efficiently on sucrose due to an increased respiration of the carbon source. Consequently, this engineered yeast strain produced less ethanol and 1.5 to 2 times more biomass when cultivated in simple batch mode using 20 g/L sucrose as the carbon source.
Conclusion: Higher cell densities during batch cultures on 20 g/L sucrose were achieved by using a S. cerevisiae strain engineered in the sucrose uptake system. Such result was accomplished by effectively reducing sucrose uptake by the yeast cells, avoiding overflow metabolism, with the concomitant reduction in ethanol production. The use of this modified yeast strain in simpler batch culture mode can be a viable option to more complicated traditional sucrose-limited fed-batch cultures for biomass-directed processes of S. cerevisiae.
Figures

Similar articles
-
Utility of an Escherichia coli strain engineered in the substrate uptake system for improved culture performance at high glucose and cell concentrations: an alternative to fed-batch cultures.Biotechnol Bioeng. 2008 Mar 1;99(4):893-901. doi: 10.1002/bit.21664. Biotechnol Bioeng. 2008. PMID: 17929322
-
Engineering topology and kinetics of sucrose metabolism in Saccharomyces cerevisiae for improved ethanol yield.Metab Eng. 2011 Nov;13(6):694-703. doi: 10.1016/j.ymben.2011.09.005. Epub 2011 Sep 22. Metab Eng. 2011. PMID: 21963484
-
Sugar utilization patterns and respiro-fermentative metabolism in the baker's yeast Torulaspora delbrueckii.Microbiology (Reading). 2007 Mar;153(Pt 3):898-904. doi: 10.1099/mic.0.2006/003475-0. Microbiology (Reading). 2007. PMID: 17322210
-
Sucrose fermentation by Saccharomyces cerevisiae lacking hexose transport.J Mol Microbiol Biotechnol. 2004;8(1):26-33. doi: 10.1159/000082078. J Mol Microbiol Biotechnol. 2004. PMID: 15741738
-
Coordination of sucrose uptake and respiration in the yeast Debaryomyces yamadae.Microbiology (Reading). 1995 Jul;141 ( Pt 7):1567-74. doi: 10.1099/13500872-141-7-1567. Microbiology (Reading). 1995. PMID: 7551025
Cited by
-
Kinetic Modeling of Saccharomyces cerevisiae Central Carbon Metabolism: Achievements, Limitations, and Opportunities.Metabolites. 2022 Jan 13;12(1):74. doi: 10.3390/metabo12010074. Metabolites. 2022. PMID: 35050196 Free PMC article. Review.
-
Privatization of public goods can cause population decline.Nat Ecol Evol. 2019 Aug;3(8):1206-1216. doi: 10.1038/s41559-019-0944-9. Epub 2019 Jul 22. Nat Ecol Evol. 2019. PMID: 31332334
-
The scientific impact of microbial cell factories.Microb Cell Fact. 2008 Dec 1;7:33. doi: 10.1186/1475-2859-7-33. Microb Cell Fact. 2008. PMID: 19046424 Free PMC article. No abstract available.
-
Elimination of sucrose transport and hydrolysis in Saccharomyces cerevisiae: a platform strain for engineering sucrose metabolism.FEMS Yeast Res. 2017 Jan 1;17(1):fox006. doi: 10.1093/femsyr/fox006. FEMS Yeast Res. 2017. PMID: 28087672 Free PMC article.
-
Controlling heterologous gene expression in yeast cell factories on different carbon substrates and across the diauxic shift: a comparison of yeast promoter activities.Microb Cell Fact. 2015 Jun 26;14:91. doi: 10.1186/s12934-015-0278-5. Microb Cell Fact. 2015. PMID: 26112740 Free PMC article.
References
-
- Bekatorou A, Psarianos C, Koutinas AA. Production of food grade yeasts. Food Technol Biotechnol. 2006;44:407–415.
-
- Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MCY, Withers ST, Shiba Y, Sarpong R, Keasling JD. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–943. doi: 10.1038/nature04640. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

