A co-fermentation strategy to consume sugar mixtures effectively
- PMID: 18304345
- PMCID: PMC2266900
- DOI: 10.1186/1754-1611-2-3
A co-fermentation strategy to consume sugar mixtures effectively
Abstract
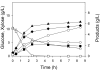
We report a new approach for the simultaneous conversion of xylose and glucose sugar mixtures into products by fermentation. The process simultaneously uses two substrate-selective strains of Escherichia coli, one which is unable to consume glucose and one which is unable to consume xylose. The xylose-selective (glucose deficient) strain E. coli ZSC113 has mutations in the glk, ptsG and manZ genes while the glucose-selective (xylose deficient) strain E. coli ALS1008 has a mutation in the xylA gene. By combining these two strains in a single process, xylose and glucose are consumed more quickly than by a single-organism approach. Moreover, we demonstrate that the process is able to adapt to changing concentrations of these two sugars, and therefore holds promise for the conversion of variable sugar feed streams, such as lignocellulosic hydrolysates.
Figures






References
-
- Sedlak M, Edenberg HJ, Ho NWY. DNA microarray analysis of the expression of the genes encoding the major enzymes in ethanol production during glucose and xylose co-fermentation by metabolically engineered Saccharomyces. Enzyme Micro Technol. 2003;33:19–28. doi: 10.1016/S0141-0229(03)00067-X. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

