Selective disruption of one Cartesian axis of cortical maps and receptive fields by deficiency in ephrin-As and structured activity
- PMID: 18304481
- PMCID: PMC2413327
- DOI: 10.1016/j.neuron.2007.12.025
Selective disruption of one Cartesian axis of cortical maps and receptive fields by deficiency in ephrin-As and structured activity
Abstract
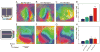
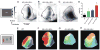
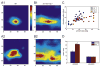
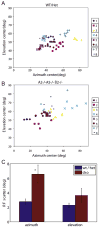
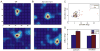
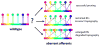
The topographic representation of visual space is preserved from retina to thalamus to cortex. We have previously shown that precise mapping of thalamocortical projections requires both molecular cues and structured retinal activity. To probe the interaction between these two mechanisms, we studied mice deficient in both ephrin-As and retinal waves. Functional and anatomical cortical maps in these mice were nearly abolished along the nasotemporal (azimuth) axis of the visual space. Both the structure of single-cell receptive fields and large-scale topography were severely distorted. These results demonstrate that ephrin-As and structured neuronal activity are two distinct pathways that mediate map formation in the visual cortex and together account almost completely for the formation of the azimuth map. Despite the dramatic disruption of azimuthal topography, the dorsoventral (elevation) map was relatively normal, indicating that the two axes of the cortical map are organized by separate mechanisms.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

