Disulfiram promotes the conversion of carcinogenic cadmium to a proteasome inhibitor with pro-apoptotic activity in human cancer cells
- PMID: 18304598
- PMCID: PMC3766637
- DOI: 10.1016/j.taap.2008.01.022
Disulfiram promotes the conversion of carcinogenic cadmium to a proteasome inhibitor with pro-apoptotic activity in human cancer cells
Abstract
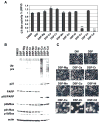
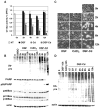
The ubiquitin-proteasome system is involved in various cellular processes, including transcription, apoptosis, and cell cycle. In vitro, in vivo, and clinical studies suggest the potential use of proteasome inhibitors as anticancer drugs. Cadmium (Cd) is a widespread environmental pollutant that has been classified as a human carcinogen. Recent study in our laboratory suggested that the clinically used anti-alcoholism drug disulfiram (DSF) could form a complex with tumor cellular copper, resulting in inhibition of the proteasomal chymotrypsin-like activity and induction of cancer cell apoptosis. In the current study, we report, for the first time, that DSF is able to convert the carcinogen Cd to a proteasome-inhibitor and cancer cell apoptosis inducer. Although the DSF-Cd complex inhibited the chymotrypsin-like activity of a purified 20S proteasome with an IC(50) value of 32 micromol/L, this complex was much more potent in inhibiting the chymotrypsin-like activity of prostate cancer cellular 26S proteasome. Inhibition of cellular proteasome activity by the DSF-Cd complex resulted in the accumulation of ubiquitinated proteins and the natural proteasome substrate p27, which was followed by activation of calpain and induction of apoptosis. Importantly, human breast cancer MCF10DCIS cells were much more sensitive to the DSF-Cd treatment than immortalized but non-tumorigenic human breast MCF-10A cells, demonstrating that the DSF-Cd complex could selectively induce proteasome inhibition and apoptosis in human tumor cells. Our work suggests the potential use of DSF for treatment of cells with accumulated levels of carcinogen Cd.
Figures



References
-
- Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004;5:417–421. - PubMed
-
- Adams J, Kauffman M. Development of the proteasome inhibitor Velcade (Bortezomib) Cancer Investig. 2004;22:304–311. - PubMed
-
- An B, Goldfarb RH, Siman R, Dou QP. Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective function and selectively accumulate the cyclin dependent kinase inhibitor p27 and induce apoptosis in transformed, but not normal, human fibroblasts. Cell Death Differ. 1998;5:1062–1075. - PubMed
-
- Beyersmann D, Hechtenberg S. Cadmium, gene regulation, and cellular signaling in mammalian cells. Toxicol Appl Pharmacol. 1997;144:247–261. - PubMed
-
- Chen D, Daniel KG, Chen MS, Kuhn DJ, Landis-Piwowar KR, Dou QP. Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem Pharmacol. 2005;69:1421–1432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

