Maternal diabetes modulates renal morphogenesis in offspring
- PMID: 18305124
- PMCID: PMC2386724
- DOI: 10.1681/ASN.2007080864
Maternal diabetes modulates renal morphogenesis in offspring
Abstract
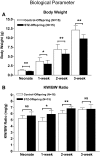
Maternal diabetes leads to an adverse in utero environment, but whether maternal diabetes impairs nephrogenesis is unknown. Diabetes was induced with streptozotocin in pregnant Hoxb7-green fluorescence protein mice at embryonic day 13, and the offspring were examined at several time points after birth. Compared with offspring of nondiabetic controls, offspring of diabetic mice had lower body weight, body size, kidney weight, and nephron number. The observed renal dysmorphogenesis may be the result of increased apoptosis, because immunohistochemical analysis revealed significantly more apoptotic podocytes as well as increased active caspase-3 immunostaining in the renal tubules compared with control mice. Regarding potential mediators of these differences, offspring of diabetic mice had increased expression of intrarenal angiotensinogen and renin mRNA, upregulation of NF-kappaB isoforms p50 and p65, and activation of the NF-kappaB pathway. In conclusion, maternal diabetes impairs nephrogenesis, possibly via enhanced intrarenal activation of the renin-angiotensin system and NF-kappaB signaling.
Figures









Comment in
-
Perinatal nephron programming is not so sweet in maternal diabetes.J Am Soc Nephrol. 2008 May;19(5):837-9. doi: 10.1681/ASN.2008030280. Epub 2008 Apr 2. J Am Soc Nephrol. 2008. PMID: 18385416 No abstract available.
References
-
- Holemans K, Aerts L, Van Assche FA: Fetal growth and long-term consequences in animal models of growth retardation. Eur J Obstet Gynecol Reprod Biol 81: 149–156, 1998 - PubMed
-
- Holemans K, Gerber RT, Meurrens K, De Clerck F, Poston L, Van Assche FA: Streptozotocin diabetes in the pregnant rat induces cardiovascular dysfunction in adult offspring. Diabetologia 42: 81–89, 1999 - PubMed
-
- Holemans K, Caluwaerts S, Van Assche FA: Unravelling the fetal origins hypothesis. Lancet 360: 2073–2075, 2002 - PubMed
-
- Holemans K, Aerts L, Van Assche FA: Fetal growth restriction and consequences for the offspring in animal models. J Soc Gynecol Investig 10: 392–399, 2003 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

