Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells
- PMID: 18305158
- PMCID: PMC2265201
- DOI: 10.1073/pnas.0712359105
Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells
Abstract
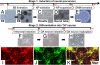
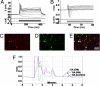
We developed a method for the efficient generation of functional dopaminergic (DA) neurons from human embryonic stem cells (hESCs) on a large scale. The most unique feature of this method is the generation of homogeneous spherical neural masses (SNMs) from the hESC-derived neural precursors. These SNMs provide several advantages: (i) they can be passaged for a long time without losing their differentiation capability into DA neurons; (ii) they can be coaxed into DA neurons at much higher efficiency than that from previous reports (86% tyrosine hydroxylase-positive neurons/total neurons); (iii) the induction of DA neurons from SNMs only takes 14 days; and (iv) no feeder cells are required during differentiation. These advantages allowed us to obtain a large number of DA neurons within a short time period and minimized potential contamination of unwanted cells or pathogens coming from the feeder layer. The highly efficient differentiation may not only enhance the efficacy of the cell therapy but also reduce the potential tumor formation from the undifferentiated residual hESCs. In line with this effect, we have never observed any tumor formation from the transplanted animals used in our study. When grafted into a parkinsonian rat model, the hESC-derived DA neurons elicited clear behavioral recovery in three behavioral tests. In summary, our study paves the way for the large-scale generation of purer and functional DA neurons for future clinical applications.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Youdim MB, Riederer P. Understanding Parkinson's disease. Sci Am. 1997;276(1):52–59. - PubMed
-
- Olanow CW, Obeso JA. Levodopa motor complications in Parkinson's disease. Ann Neurol. 2000;47:167–178. - PubMed
-
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. - PubMed
-
- Desbaillets I, Ziegler U, Groscurth P, Gassmann M. Embryoid bodies: An in vitro model of mouse embryogenesis. Exp Physiol. 2000;85:645–651. - PubMed
-
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

