Yeast Pch2 promotes domainal axis organization, timely recombination progression, and arrest of defective recombinosomes during meiosis
- PMID: 18305165
- PMCID: PMC2265181
- DOI: 10.1073/pnas.0711864105
Yeast Pch2 promotes domainal axis organization, timely recombination progression, and arrest of defective recombinosomes during meiosis
Abstract
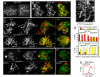
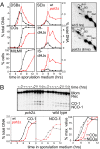
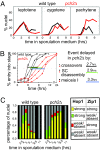
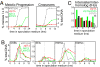
We show that, during budding yeast meiosis, axis ensemble Hop1/Red1 and synaptonemal complex (SC) component Zip1 tend to occur in alternating strongly staining domains. The widely conserved AAA+-ATPase Pch2 mediates this pattern, likely by means of direct intervention along axes. Pch2 also coordinately promotes timely progression of cross-over (CO) and noncross-over (NCO) recombination. Oppositely, in a checkpoint-triggering aberrant situation (zip1Delta), Pch2 mediates robust arrest of stalled recombination complexes, likely via nucleolar localization. We suggest that, during WT meiosis, Pch2 promotes progression of SC-associated CO and NCO recombination complexes at a regulated early-midpachytene transition that is rate-limiting for later events; in contrast, during defective meiosis, Pch2 ensures that aberrant recombination complexes fail to progress so that intermediates can be harmlessly repaired during eventual return to growth. Positive vs. negative roles of Pch2 in the two situations are analogous to positive vs. negative roles of Mec1/ATR, suggesting that Pch2 might mediate Mec1/ATR activity. We further propose that regulatory surveillance of normal and abnormal interchromosomal interactions in mitotic and meiotic cells may involve "structure-dependent interchromosomal interaction" (SDIX) checkpoints.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sym M, Engebrecht JA, Roeder GS. ZIP1 is a synaptonemal complex protein required for meiotic chromosome synapsis. Cell. 1993;72:365–378. - PubMed
-
- Blat Y, Protacio RU, Hunter N, Kleckner N. Physical and functional interactions among basic chromosome organizational features govern early steps of meiotic chiasma formation. Cell. 2002;111:791–802. - PubMed
-
- Keeney S. Mechanism and control of meiotic recombination initiation. Curr Top Dev Biol. 2001;52:1–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

