Internal body state influences topographical plasticity of sensory representations in the rat gustatory cortex
- PMID: 18305172
- PMCID: PMC2268816
- DOI: 10.1073/pnas.0708927105
Internal body state influences topographical plasticity of sensory representations in the rat gustatory cortex
Abstract
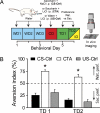
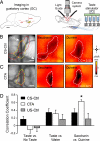
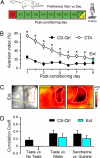
Primary sensory cortices are remarkably organized in spatial maps according to specific sensory features of the stimuli. These cortical maps can undergo plastic rearrangements after changes in afferent ("bottom-up") sensory inputs such as peripheral lesions or passive sensory experience. However, much less is known about the influence of "top-down" factors on cortical plasticity. Here, we studied the effect of a visceral malaise on taste representations in the gustatory cortex (GC). Using in vivo optical imaging, we showed that inducing conditioned taste aversion (CTA) to a sweet and pleasant stimulus induced plastic rearrangement of its cortical representation, becoming more similar to a bitter and unpleasant taste representation. Using a behavior task, we showed that changes in hedonic perception are directly related to the maps plasticity in the GC. Indeed imaging the animals after CTA extinction indicated that sweet and bitter representations were dissimilar. In conclusion, we showed that an internal state of malaise induces plastic reshaping in the GC associated to behavioral shift of the stimulus hedonic value. We propose that the GC not only encodes taste modality, intensity, and memory but extends its integrative properties to process also the stimulus hedonic value.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Buonomano DV, Merzenich MM. Cortical plasticity: From synapses to maps. Annu Rev Neurosci. 1998;21:149–186. - PubMed
-
- Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–815. - PubMed
-
- Polley DB, Kvasnak E, Frostig RD. Naturalistic experience transforms sensory maps in the adult cortex of caged animals. Nature. 2004;429:67–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

