MHC class II stabilization at the surface of human dendritic cells is the result of maturation-dependent MARCH I down-regulation
- PMID: 18305173
- PMCID: PMC2265198
- DOI: 10.1073/pnas.0708874105
MHC class II stabilization at the surface of human dendritic cells is the result of maturation-dependent MARCH I down-regulation
Abstract
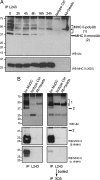
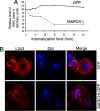
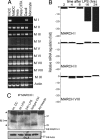
In response to Toll-like receptor ligands, dendritic cells (DCs) dramatically enhance their antigen presentation capacity by stabilizing at the cell-surface MHC II molecules. We demonstrate here that, in human monocyte-derived DCs, the RING-CH ubiquitin E3 ligase, membrane-associated RING-CH I (MARCH I), promotes the ubiquitination of the HLA-DR beta-chain. Thus, in nonactivated DCs, MARCH I induces the surface internalization of mature HLA-DR complexes, therefore reducing their stability and levels. We further demonstrate that the maturation-dependent down-regulation of MARCH I is a key event in MHC class II up-regulation at the surface of LPS-activated DCs. MARCH I is, therefore, a major regulator of HLA-DR traffic, and its loss contributes to the acquisition of the potent immunostimulatory properties of mature human DCs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

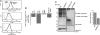
References
-
- Mellman I, Steinman RM. Cell. 2001;106:255–258. - PubMed
-
- Reis e Sousa C. Nat Rev Immunol. 2006;6:476–483. - PubMed
-
- Pierre P, Turley SJ, Gatti E, Hull M, Meltzer J, Misra A, Inaba K, Steinman RM, Mellman I. Nature. 1997;388:787–792. - PubMed
-
- van Niel G, Wubbolts R, Ten Broeke T, Buschow SI, Ossendorp FA, Melief CJ, Raposo G, van Balkom BW, Stoorvogel W. Immunity. 2006;25:885–894. - PubMed
-
- Shin JS, Ebersold M, Pypaert M, Delamarre L, Hartley A, Mellman I. Nature. 2006;444:115–118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

