Regulation of synaptic inhibition by phospho-dependent binding of the AP2 complex to a YECL motif in the GABAA receptor gamma2 subunit
- PMID: 18305175
- PMCID: PMC2265186
- DOI: 10.1073/pnas.0707920105
Regulation of synaptic inhibition by phospho-dependent binding of the AP2 complex to a YECL motif in the GABAA receptor gamma2 subunit
Abstract
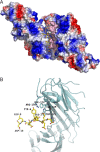
The regulation of the number of gamma2-subunit-containing GABA(A) receptors (GABA(A)Rs) present at synapses is critical for correct synaptic inhibition and animal behavior. This regulation occurs, in part, by the controlled removal of receptors from the membrane in clathrin-coated vesicles, but it remains unclear how clathrin recruitment to surface gamma2-subunit-containing GABA(A)Rs is regulated. Here, we identify a gamma2-subunit-specific Yxxvarphi-type-binding motif for the clathrin adaptor protein, AP2, which is located within a site for gamma2-subunit tyrosine phosphorylation. Blocking GABA(A)R-AP2 interactions via this motif increases synaptic responses within minutes. Crystallographic and biochemical studies reveal that phosphorylation of the Yxxvarphi motif inhibits AP2 binding, leading to increased surface receptor number. In addition, the crystal structure provides an explanation for the high affinity of this motif for AP2 and suggests that gamma2-subunit-containing heteromeric GABA(A)Rs may be internalized as dimers or multimers. These data define a mechanism for tyrosine kinase regulation of GABA(A)R surface levels and synaptic inhibition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Kittler JT, McAinsh K, Moss SJ. Mol Neurobiol. 2002;26:251–268. - PubMed
-
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Nat Neurosci. 1998;1:563–571. - PubMed
-
- Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, Belzung C, Fritschy JM, Luscher B, Mohler H. Nat Neurosci. 1999;2:833–839. - PubMed
-
- Moss SJ, Gorrie GH, Amato A, Smart TG. Nature. 1995;377:344–348. - PubMed
-
- Brandon NJ, Delmas P, Hill J, Smart TG, Moss SJ. Neuropharmacology. 2001;41:745–752. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- NS056359/NS/NINDS NIH HHS/United States
- R01 NS048045/NS/NINDS NIH HHS/United States
- NS048045/NS/NINDS NIH HHS/United States
- R01 NS046478/NS/NINDS NIH HHS/United States
- P01 NS054900/NS/NINDS NIH HHS/United States
- NS046478/NS/NINDS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 NS056359/NS/NINDS NIH HHS/United States
- P01NS054900/NS/NINDS NIH HHS/United States
- R01 NS047478/NS/NINDS NIH HHS/United States
- R01 NS051195/NS/NINDS NIH HHS/United States
- G120/972/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

