Cu(II) organizes beta-2-microglobulin oligomers but is released upon amyloid formation
- PMID: 18305198
- PMCID: PMC2271169
- DOI: 10.1110/ps.073249008
Cu(II) organizes beta-2-microglobulin oligomers but is released upon amyloid formation
Abstract
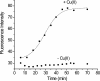
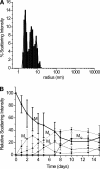
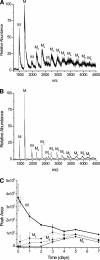
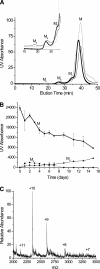
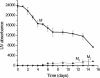
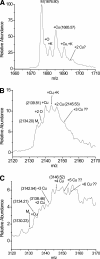
beta-2-Microglobulin (beta2m) is deposited as amyloid fibrils in the bones and joints of patients undergoing long-term dialysis treatment as a result of kidney failure. Previous work has shown that biologically relevant amounts of Cu(II) can cause beta2m to be converted to amyloid fibrils under physiological conditions in vitro. In this work, dynamic light scattering, mass spectrometry, and size-exclusion chromatography are used to characterize the role that Cu plays in the formation of oligomeric intermediates that precede fibril formation. Cu(II) is found to be necessary for the stability of the dimer and an initial form of the tetramer. The initially formed tetramer then undergoes a structural change to a state that no longer binds Cu(II) before progressing to a hexameric state. Based on these results, we propose that the lag phase associated with beta2m fibril formation is partially accounted for by the structural transition of the tetramer that results in Cu(II) loss. Consistent with this observation is the determination that the mature beta2m amyloid fibrils do not contain Cu. Thus, Cu(II) appears to play a catalytic role by enabling the organization of the necessary oligomeric intermediates that precede beta2m amyloid formation.
Figures

References
-
- Bjorkman, P.J., Saper, M.A., Samraoui, B., Bennett, W.S., Strominger, J.L., Wiley, D.C. Structure of the human class-I histocompatibility antigen, HLA-A2. Nature. 1987;329:506–512. - PubMed
-
- Calabrese, M.F., Miranker, A.D. Formation of a stable oligomer of β-2 microglobulin requires only transient encounter with Cu(II) J. Mol. Biol. 2007;367:1–7. - PubMed
-
- Connors, L.H., Shirahama, T., Skinner, M., Fenves, A., Cohen, A.S. In vitro formation of amyloid fibrils from intact β-2-microglobulin. Biochem. Biophys. Res. Commun. 1985;131:1063–1068. - PubMed
-
- Davis, D.P., Gallo, G., Vogen, S.M., Dul, J.L., Sciarretta, K.L., Kumar, A., Raffen, R., Stevens, F.J., Argon, Y. Both the environment and somatic mutations govern the aggregation pathway of pathogenic immunoglobulin light chain. J. Mol. Biol. 2001;313:1021–1034. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

