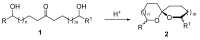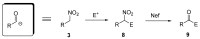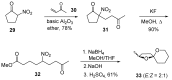Nitroalkanes as central reagents in the synthesis of spiroketals
- PMID: 18305420
- PMCID: PMC6244950
- DOI: 10.3390/molecules13020319
Nitroalkanes as central reagents in the synthesis of spiroketals
Abstract
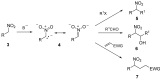
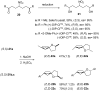
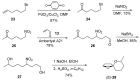
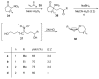
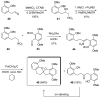
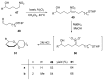
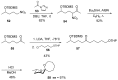
Nitroalkanes can be profitably employed as carbanionic precursors for the assembly of dihydroxy ketone frameworks, suitable for the preparation of spiroketals. The carbon-carbon bond formation is carried out exploiting nitroaldol and Michael reactions, while the nitro to carbonyl conversion (Nef reaction) ensures the correct introduction of the keto group. Several spiroketal systems endowed with considerable biological activity can be prepared using this synthetic strategy.
Figures
References
-
- Perron F., Albizati K. F. Chemistry of Spiroketals. Chem. Rev. 1989;89:1617–1661. doi: 10.1021/cr00097a015. - DOI
-
- Brimble M. A., Farès A. F. Synthesis of Bis-Spiroacetals Ring Systems. Tetrahedron. 1999;55:7661–7706. doi: 10.1016/S0040-4020(99)00387-7. - DOI
-
- Franke W., Kitching W. Spiroacetals in Insects. Curr. Org. Chem. 2001;5:233–251. doi: 10.2174/1385272013375652. - DOI
-
- Rodríguez S., Wipf P. Oxidative Spiroacetalizations and Spirolactonizations of Arenes. Synthesis. 2004:2767–2783.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources