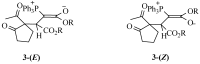A facile route to diastereomeric phosphorus ylides. Chemoselective synthesis of dialkyl (E)-2-[1-(2-oxocyclopentylidene)ethyl]-2-butenedioates
- PMID: 18305421
- PMCID: PMC6245385
- DOI: 10.3390/molecules13020331
A facile route to diastereomeric phosphorus ylides. Chemoselective synthesis of dialkyl (E)-2-[1-(2-oxocyclopentylidene)ethyl]-2-butenedioates
Abstract
2-Acetylcyclopentanone undergoes a smooth reaction with triphenylphosphine and dialkyl acetylenedicarboxylates to produce dialkyl 2-(1-acetyl-2-oxocyclopentyl)-3-(1,1,1-triphenyl-lambda(5)-phosphanylidene)succinates. These compounds undergo intramolecular Wittig reactions in boiling benzene to produce highly strained spirocyclobutene derivatives, which spontaneously undergo ring-opening reactions to produce dialkyl (E)-2-[1-(2-oxocyclopentyliden)ethyl]-2-butenedioates.
Similar articles
-
Catalyst-free synthesis of skipped dienes from phosphorus ylides, allylic carbonates, and aldehydes via a one-pot SN2' allylation-Wittig strategy.J Org Chem. 2014 Apr 18;79(8):3696-703. doi: 10.1021/jo500375q. Epub 2014 Apr 2. J Org Chem. 2014. PMID: 24661220
-
Olefination reactions of phosphorus-stabilized carbon nucleophiles.Top Curr Chem. 2012;327:197-238. doi: 10.1007/128_2012_314. Top Curr Chem. 2012. PMID: 22371171 Review.
-
A highly tunable stereoselective olefination of semistabilized triphenylphosphonium ylides with N-sulfonyl imines.J Am Chem Soc. 2010 Apr 14;132(14):5018-20. doi: 10.1021/ja910238f. J Am Chem Soc. 2010. PMID: 20302297
-
Single-Step Construction of the anti-Deoxypropionate Motif from Propylene: Formal Total Synthesis of the Cuticular Hydrocarbons Isolated from Antitrogus parvulus.Angew Chem Int Ed Engl. 2018 Aug 27;57(35):11394-11398. doi: 10.1002/anie.201804711. Epub 2018 Jul 26. Angew Chem Int Ed Engl. 2018. PMID: 29953709
-
Asymmetric hydrogenation of minimally functionalised terminal olefins: an alternative sustainable and direct strategy for preparing enantioenriched hydrocarbons.Chemistry. 2010 Dec 27;16(48):14232-40. doi: 10.1002/chem.201001909. Chemistry. 2010. PMID: 21140401 Review.
References
-
- Hudson H. R. In: The Chemistry of Organophosphorus Compounds: Primary, Secondary and Tertiary Phosphines and Heterocyclic Organophosphorus (III) Compounds. Hantely F. R., editor. Vol. 1. Wiley; New York: 1990. pp. 386–472.
-
- Shen Y. New Synthetic Methodologies for Carbon-Carbon Double Bond Formation. Acc. Chem Re. 1998;31:584–592.
-
- Puke C., Erker G., Aust N. C., Wurthwein E. V., Frohlich R. Evidence for a Continuous Transition between Thiaphosphetane and Betaine-Type Structures in the Thio-Wittig Reaction. J. Am. Chem. Soc. 1998;120:4863–4864. doi: 10.1021/ja9720669. - DOI
-
- Corbridge D. E. C. Phosphorus: An Outline of Chemistry, Biochemistry and Uses. 5th ed. Elsevier; Amsterdam: 1995. pp. a) pp. 42-47; b) pp. 319-323; c) pp. 361-362.
-
- Cadogan J. I. G. Organophosphorus Reagents in Organic Synthesis. Academic Press; New York: 1979.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources