Structure and catalytic properties of carboxylesterase isozymes involved in metabolic activation of prodrugs
- PMID: 18305428
- PMCID: PMC6245361
- DOI: 10.3390/molecules13020412
Structure and catalytic properties of carboxylesterase isozymes involved in metabolic activation of prodrugs
Abstract
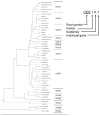
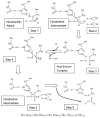
Mammalian carboxylesterases (CESs) comprise a multigene family whose gene products play important roles in biotransformation of ester- or amide-type prodrugs. They are members of an alpha,beta-hydrolase-fold family and are found in various mammals. It has been suggested that CESs can be classified into five major groups denominated CES1-CES5, according to the homology of the amino acid sequence, and the majority of CESs that have been identified belong to the CES1 or CES2 family. The substrate specificities of CES1 and CES2 are significantly different. The CES1 isozyme mainly hydrolyzes a substrate with as mall alcohol group and large acyl group, but its wide active pocket sometimes allows it to act on structurally distinct compounds of either a large or small alcohol moiety. In contrast, the CES2 isozyme recognizes a substrate with a large alcohol group and small acyl group, and its substrate specificity may be restricted by the capability of acyl-enzyme conjugate formation due to the presence of conformational interference in the active pocket. Since pharmacokinetic and pharmacological data for prodrugs obtained from preclinical experiments using various animals are generally used as references for human studies, it is important to clarify the biochemical properties of CES isozymes. Further experimentation for an understanding of detailed substrate specificity of prodrugs for CES isozymes and its hydrolysates will help us to design the ideal prodrugs.
Figures


References
-
- Furihata T., Hosokawa M., Koyano N., Nakamura T., Satoh T., Chiba K. Identification of di-(2-ethylhexyl) phthalate-induced carboxylesterase 1 in C57BL/6 mouse liver microsomes: purification, cDNA cloning, and baculovirus-mediated expression. Drug Metab. Dispos. 2004;32:1170–7. doi: 10.1124/dmd.104.000620. - DOI - PubMed
-
- Furihata T., Hosokawa M., Nakata F., Satoh T., Chiba K. Purification, molecular cloning, and functional expression of inducible liver acylcarnitine hydrolase in C57BL/6 mouse, belonging to the carboxylesterase multigene family. Arch. Biochem. Biophys. 2003;416:101–9. doi: 10.1016/S0003-9861(03)00286-8. - DOI - PubMed
-
- Hosokawa M., Satoh T. Molecular aspect of the inter-species variation in carboxylesterase, 7th North American ISSX Meeting; San Diego, CA, USA. 1996.
-
- Hosokawa M., Suzuki K., Takahashi D., Mori M., Satoh T., Chiba K. Purification, molecular cloning, and functional expression of dog liver microsomal acyl-CoA hydrolase: a member of the carboxylesterase multigene family. Arch. Biochem. Biophys. 2001;389:245–53. doi: 10.1006/abbi.2001.2346. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

