The human endonuclease III enzyme is a relevant target to potentiate cisplatin cytotoxicity in Y-box-binding protein-1 overexpressing tumor cells
- PMID: 18307537
- PMCID: PMC11159512
- DOI: 10.1111/j.1349-7006.2008.00739.x
The human endonuclease III enzyme is a relevant target to potentiate cisplatin cytotoxicity in Y-box-binding protein-1 overexpressing tumor cells
Abstract
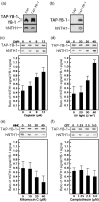
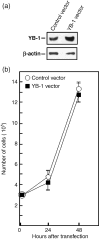
Y-box-binding protein-1 (YB-1) is a multifunctional protein involved in the regulation of transcription, translation, and mRNA splicing. In recent years, several laboratories have demonstrated that YB-1 is directly involved in the cellular response to genotoxic stress. Importantly, YB-1 is increased in tumor cell lines resistant to cisplatin, and the level of nuclear expression of YB-1 is predictive of drug resistance and patient outcome in breast tumors, ovarian cancers, and synovial sarcomas. YB-1 binds to several DNA repair enzymes in vitro including human endonuclease III (hNTH1). Human NTH1 is a bifunctional DNA glycosylase/apurinic/apyrimidinic lyase involved in base excision repair. In this study, we show that YB-1 binds specifically to the auto-inhibitory domain of hNTH1, providing a mechanism by which YB-1 stimulates hNTH1 activity. Indeed, YB-1 strongly stimulates in vitro the activity of hNTH1 toward DNA duplex probes containing oxidized bases, lesions prone to be present in cisplatin treated cells. We also observed an increase in YB-1/hNTH1 complex formation in the mammary adenocarcinoma MCF7 cell line treated with UV light and cisplatin. Such an increase was not observed with mitomycin C or the topoisomerase I inhibitor camptothecin. Accordingly, antisense RNAs against either YB-1 or hNTH1 increased cellular sensitivity to UV and cisplatin but not to mitomycin C. An antisense RNA against YB-1 increased camptothecin sensitivity. In contrast, an antisense against hNTH1 did not. Finally, siRNA against hNTH1 re-established cytotoxicity in otherwise cisplatin-resistant YB-1 overexpressing MCF7 cells. These data indicate that hNTH1 is a relevant target to potentiate cisplatin cytotoxicity in YB-1 overexpressing tumor cells.
Figures





Similar articles
-
Förster Resonance Energy Transfer Based Biosensor for Targeting the hNTH1-YB1 Interface as a Potential Anticancer Drug Target.ACS Chem Biol. 2020 Apr 17;15(4):990-1003. doi: 10.1021/acschembio.9b01023. Epub 2020 Mar 17. ACS Chem Biol. 2020. PMID: 32125823
-
Stimulation of human endonuclease III by Y box-binding protein 1 (DNA-binding protein B). Interaction between a base excision repair enzyme and a transcription factor.J Biol Chem. 2001 Jun 15;276(24):21242-9. doi: 10.1074/jbc.M101594200. Epub 2001 Apr 3. J Biol Chem. 2001. PMID: 11287425
-
Substrate specificity of human endonuclease III (hNTH1). Effect of human APE1 on hNTH1 activity.J Biol Chem. 2003 Mar 14;278(11):9005-12. doi: 10.1074/jbc.M212168200. Epub 2003 Jan 8. J Biol Chem. 2003. PMID: 12519758
-
[Molecular mechanism of the stress induction of MDR1 gene].Nihon Rinsho. 1997 May;55(5):1054-8. Nihon Rinsho. 1997. PMID: 9155152 Review. Japanese.
-
Potential Therapeutic Strategies for Targeting Y-Box-Binding Protein 1 in Cancers.Curr Cancer Drug Targets. 2021;21(11):897-906. doi: 10.2174/1568009621666210831125001. Curr Cancer Drug Targets. 2021. PMID: 34465278 Review.
Cited by
-
An integrative approach to identify YB-1-interacting proteins required for cisplatin resistance in MCF7 and MDA-MB-231 breast cancer cells.Cancer Sci. 2011 Jul;102(7):1410-7. doi: 10.1111/j.1349-7006.2011.01948.x. Epub 2011 May 5. Cancer Sci. 2011. PMID: 21466612 Free PMC article.
-
Y-box binding protein 1 enhances DNA topoisomerase 1 activity and sensitivity to camptothecin via direct interaction.J Exp Clin Cancer Res. 2014 Dec 24;33(1):112. doi: 10.1186/s13046-014-0112-7. J Exp Clin Cancer Res. 2014. PMID: 25539742 Free PMC article.
-
Regulation of Poly(ADP-Ribose) Polymerase 1 Activity by Y-Box-Binding Protein 1.Biomolecules. 2020 Sep 16;10(9):1325. doi: 10.3390/biom10091325. Biomolecules. 2020. PMID: 32947956 Free PMC article.
-
Prognostic role of YB-1 expression in breast cancer: a meta-analysis.Int J Clin Exp Med. 2015 Feb 15;8(2):1780-91. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 25932106 Free PMC article.
-
Upregulation of human DNA binding protein A (dbpA) in gastric cancer cells.Acta Pharmacol Sin. 2009 Oct;30(10):1436-42. doi: 10.1038/aps.2009.137. Epub 2009 Sep 14. Acta Pharmacol Sin. 2009. PMID: 19749785 Free PMC article.
References
-
- Swamynathan SK, Nambiar A, Guntaka RV. Role of single‐stranded DNA regions and Y‐box proteins in transcriptional regulation of viral and cellular genes. FASEB J 1998; 12: 515–22. - PubMed
-
- Hayakawa H, Uchiumi T, Fukuda T et al . Binding capacity of human YB‐1 protein for RNA containing 8‐oxoguanine. Biochemistry 2002; 41: 12739–44. - PubMed
-
- Koike K, Uchiumi T, Ohga T et al . Nuclear translocation of the Y‐box binding protein by ultraviolet irradiation. FEBS Lett 1997; 417: 390–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

