An optimized ibuprofen dosing scheme for preterm neonates with patent ductus arteriosus, based on a population pharmacokinetic and pharmacodynamic study
- PMID: 18307541
- PMCID: PMC2432471
- DOI: 10.1111/j.1365-2125.2008.03118.x
An optimized ibuprofen dosing scheme for preterm neonates with patent ductus arteriosus, based on a population pharmacokinetic and pharmacodynamic study
Abstract
What is already known about this subject: Ibuprofen is a nonsteroidal anti-inflammatory agent that induces closure of the patent ductus arteriosus in neonates. Few studies of ibuprofen pharmacokinetics have been performed and were limited to small groups of preterm infants, showing a large intersubject variability and an increase in clearance with either postnatal or gestational age.
What this study adds: A population pharmacokinetic study was performed on 66 neonates to characterize the concentration-time courses of ibuprofen. Ibuprofen clearance significantly increased from postnatal age day 1 to day 8, but not with gestational age. A relationship was shown between ibuprofen area under the curve (AUC) and patent ductus arteriosus closure rate, and an effective threshold AUC was evidenced. Dosing schemes were proposed as a function of postnatal age, to achieve this AUC and to improve the efficacy of treatment for patent ductus arteriosus in neonates. AIMS To describe ibuprofen pharmacokinetics in preterm neonates with patent ductus arteriosus (PDA) and to establish relationships between doses, plasma concentrations and ibuprofen efficacy and safety.
Methods: Sixty-six neonates were treated with median daily doses of 10, 5 and 5 mg kg(-1) of ibuprofen-lysine by intravenous infusion on 3 consecutive days. A population pharmacokinetic model was developed with NONMEM. Bayesian individual pharmacokinetic estimates were used to calculate areas under the curve (AUC) and to simulate doses. A logistic regression was performed on PDA closure.
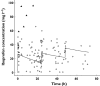
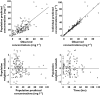
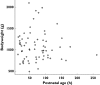
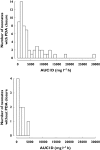
Results: Ibuprofen pharmacokinetics were described by a one-compartment model with linear elimination. Mean population pharmacokinetic estimates with corresponding intersubject variabilities (%) were: elimination clearance CL = 9.49 ml h(-1) (62%) and volume of distribution V = 375 ml (72%). Ibuprofen CL significantly increased with postnatal age (PNA): CL = 9.49*(PNA/96.3)(1.49). AUC after the first dose (AUC1D), the sum of AUC after the three doses (AUC3D) and gestational age were significantly higher in 57 neonates with closing PDA than in nine neonates without PDA closure (P = 0.02). PDA closure was observed in 50% of the neonates when AUC1D < 600 mg l(-1) h (or AUC3D < 900 mg l(-1) h) and in 91% when AUC1D > 600 mg l(-1) h (or AUC3D > 900 mg l(-1) h) (P = 0.006). No correlation between AUC and side-effects could be demonstrated.
Conclusions: To achieve these optimal AUCs, irrespective of gestational age, three administrations at 24 h intervals are recommended of 10, 5, 5 mg kg(-1) for neonates younger than 70 h, 14, 7, 7 mg kg(-1) for neonates between 70 and 108 h and 18, 9, 9 mg kg(-1) for neonates between 108 and 180 h.
Figures
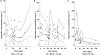
References
-
- Hermes-DeSantis ER, Clymann RI. Patent ductus arteriosus: pathophysiology and management. J Perinatol. 2006;26:S14–8. S22. 3. - PubMed
-
- Van Overmeire B, Chemtob S. The pharmacologic closure of the patent ductus arteriosus. Semin Fetal Neonatal Med. 2005;10:177–84. - PubMed
-
- Adams SS, Bresloff P, Mason CG. Pharmacological differences between the optical isomers of ibuprofen: evidence for metabolic inversion of the (-) -isomer. J Pharm Pharmacol. 1976;28:256–7. - PubMed
-
- Van Overmeire B, Smets K, Lecoutere D, Van de Broek H, Weyler J, Degroote K, Langhendries JP. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2000;343:674–81. - PubMed
-
- Aranda JV, Varvarigou A, Beharry K, Bansal R, Bardin C, Modanlou H, Papageorgiou A, Chemtob S. Pharmacokinetics and protein binding of intravenous ibuprofen in the premature newborn infant. Acta Paediatr. 1997;86:289–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

