Reliability and applications of statistical methods based on oligonucleotide frequencies in bacterial and archaeal genomes
- PMID: 18307761
- PMCID: PMC2289816
- DOI: 10.1186/1471-2164-9-104
Reliability and applications of statistical methods based on oligonucleotide frequencies in bacterial and archaeal genomes
Abstract
Background: The increasing number of sequenced prokaryotic genomes contains a wealth of genomic data that needs to be effectively analysed. A set of statistical tools exists for such analysis, but their strengths and weaknesses have not been fully explored. The statistical methods we are concerned with here are mainly used to examine similarities between archaeal and bacterial DNA from different genomes. These methods compare observed genomic frequencies of fixed-sized oligonucleotides with expected values, which can be determined by genomic nucleotide content, smaller oligonucleotide frequencies, or be based on specific statistical distributions. Advantages with these statistical methods include measurements of phylogenetic relationship with relatively small pieces of DNA sampled from almost anywhere within genomes, detection of foreign/conserved DNA, and homology searches. Our aim was to explore the reliability and best suited applications for some popular methods, which include relative oligonucleotide frequencies (ROF), di- to hexanucleotide zero'th order Markov methods (ZOM) and 2.order Markov chain Method (MCM). Tests were performed on distant homology searches with large DNA sequences, detection of foreign/conserved DNA, and plasmid-host similarity comparisons. Additionally, the reliability of the methods was tested by comparing both real and random genomic DNA.
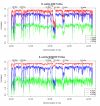
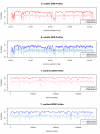
Results: Our findings show that the optimal method is context dependent. ROFs were best suited for distant homology searches, whilst the hexanucleotide ZOM and MCM measures were more reliable measures in terms of phylogeny. The dinucleotide ZOM method produced high correlation values when used to compare real genomes to an artificially constructed random genome with similar %GC, and should therefore be used with care. The tetranucleotide ZOM measure was a good measure to detect horizontally transferred regions, and when used to compare the phylogenetic relationships between plasmids and hosts, significant correlation (R2 = 0.4) was found with genomic GC content and intra-chromosomal homogeneity.
Conclusion: The statistical methods examined are fast, easy to implement, and powerful for a number of different applications involving genomic sequence comparisons. However, none of the measures examined were superior in all tests, and therefore the choice of the statistical method should depend on the task at hand.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

