Array-based gene discovery with three unrelated subjects shows SCARB2/LIMP-2 deficiency causes myoclonus epilepsy and glomerulosclerosis
- PMID: 18308289
- PMCID: PMC2427287
- DOI: 10.1016/j.ajhg.2007.12.019
Array-based gene discovery with three unrelated subjects shows SCARB2/LIMP-2 deficiency causes myoclonus epilepsy and glomerulosclerosis
Abstract
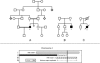
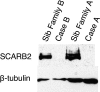
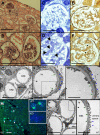
Action myoclonus-renal failure syndrome (AMRF) is an autosomal-recessive disorder with the remarkable combination of focal glomerulosclerosis, frequently with glomerular collapse, and progressive myoclonus epilepsy associated with storage material in the brain. Here, we employed a novel combination of molecular strategies to find the responsible gene and show its effects in an animal model. Utilizing only three unrelated affected individuals and their relatives, we used homozygosity mapping with single-nucleotide polymorphism chips to localize AMRF. We then used microarray-expression analysis to prioritize candidates prior to sequencing. The disorder was mapped to 4q13-21, and microarray-expression analysis identified SCARB2/Limp2, which encodes a lysosomal-membrane protein, as the likely candidate. Mutations in SCARB2/Limp2 were found in all three families used for mapping and subsequently confirmed in two other unrelated AMRF families. The mutations were associated with lack of SCARB2 protein. Reanalysis of an existing Limp2 knockout mouse showed intracellular inclusions in cerebral and cerebellar cortex, and the kidneys showed subtle glomerular changes. This study highlights that recessive genes can be identified with a very small number of subjects. The ancestral lysosomal-membrane protein SCARB2/LIMP-2 is responsible for AMRF. The heterogeneous pathology in the kidney and brain suggests that SCARB2/Limp2 has pleiotropic effects that may be relevant to understanding the pathogenesis of other forms of glomerulosclerosis or collapse and myoclonic epilepsies.
Figures



References
-
- Andermann E., Andermann F., Carpenter S., Wolfe L.S., Nelson R., Patry G., Boileau J. Action myoclonus-renal failure syndrome: A previously unrecognized neurological disorder unmasked by advances in nephrology. Adv. Neurol. 1986;43:87–103. - PubMed
-
- Badhwar A., Berkovic S.F., Dowling J.P., Gonzales M., Narayanan S., Brodtmann A., Berzen L., Caviness J., Trenkwalder C., Winkelmann J. Action myoclonus-renal failure syndrome: Characterization of a unique cerebro-renal disorder. Brain. 2004;127:2173–2182. - PubMed
-
- Vadlamudi L., Vears D.F., Hughes A., Pedagogus E., Berkovic S.F. Action myoclonus-renal failure syndrome: A cause for worsening tremor in young adults. Neurology. 2006;67:1310–1311. - PubMed
-
- Di X., Matsuzaki H., Webster T.A., Hubbell E., Liu G., Dong S., Bartell D., Huang J., Chiles R., Yang G. Dynamic model based algorithms for screening and genotyping over 100 K SNPs on oligonucleotide microarrays. Bioinformatics. 2005;21:1958–1963. - PubMed
-
- Terwilliger J.D., Ott J. Johns Hopkins University Press; Baltimore, London: 1994. Handbook of Human Genetic Linkage.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

