Prevention of bone loss after withdrawal of tamoxifen
- PMID: 18308653
- PMCID: PMC3140137
- DOI: 10.4158/EP.14.2.162
Prevention of bone loss after withdrawal of tamoxifen
Abstract
Objective: Tamoxifen has antiestrogenic effects in the breast and estrogenlike activity in the skeletons of postmenopausal women. We hypothesized that postmenopausal women with breast cancer would experience a rapid decline in bone mineral density (BMD) after stopping tamoxifen, similar to that seen with estrogen withdrawal. The objective of this study was to assess, in a randomized, double-blind, placebo-controlled trial, whether administration of alendronate (70 mg weekly) would prevent bone loss associated with tamoxifen discontinuation.
Methods: Postmenopausal women with breast cancer were randomly assigned to receive alendronate or placebo for 1 year within 3 months after withdrawal of tamoxifen therapy. We initiated a randomized, double-blind, placebo-controlled trial of alendronate (70 mg weekly) in an effort to prevent bone loss associated with discontinuation of tamoxifen therapy. Patients treated with aromatase inhibitors were excluded from the study. BMD at the spine, hip, and forearm was measured at baseline and at 12 months. Analyses employed repeated-measures analysis of variance.
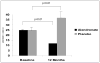
Results: Patient accrual was considerably limited by the substantial increase in use of aromatase inhibitors during the enrollment period. The study patients (N = 11) had similar baseline BMD T-scores in the alendronate (n = 6) and placebo (n = 5) subgroups. After 1 year, tamoxifen withdrawal was associated with a significant decline in BMD at the femoral neck, which appeared to be prevented by weekly administration of alendronate (-5.2% versus 0.1%; P = .02). Levels of urinary N-telopeptide, a marker of bone turnover, increased by 48% in study subjects in the placebo group (P < .01), whereas weekly alendronate treatment was associated with a 52% decline (P < .01) in this bone resorption marker.
Conclusion: Differences in BMD and bone turnover were evident despite the small sample size. These data suggest that postmenopausal women with breast cancer completing tamoxifen therapy warrant an evaluation of their skeletal health and that bisphosphonate therapy may be useful in preventing bone loss associated with discontinuation of tamoxifen.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


References
-
- Delmas PD, Fontana A. Bone loss induced by cancer treatment and its management. Eur J Cancer. 1998;34:260–262. - PubMed
-
- Valagussa P, Moliterni A, Zambetti M, Bonadonna G. Long-term sequelae from adjuvant chemotherapy. Recent Results Cancer Res. 1993;127:247–255. - PubMed
-
- Minton SE, Munster PN. Chemotherapy-induced amenorrhea and fertility in women undergoing adjuvant treatment for breast cancer. Cancer Control. 2002;9:466–472. - PubMed
-
- Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy pre-menopausal and postmenopausal women. J Clin Oncol. 1996;14:78–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

