Low protein diet fed exclusively during mouse oocyte maturation leads to behavioural and cardiovascular abnormalities in offspring
- PMID: 18308825
- PMCID: PMC2465188
- DOI: 10.1113/jphysiol.2007.149229
Low protein diet fed exclusively during mouse oocyte maturation leads to behavioural and cardiovascular abnormalities in offspring
Abstract
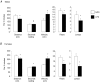
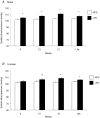
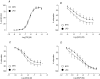
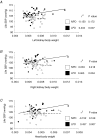
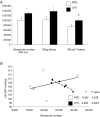
Early embryonic development is known to be susceptible to maternal undernutrition, leading to a disease-related postnatal phenotype. To determine whether this sensitivity extended into oocyte development, we examined the effect of maternal normal protein diet (18% casein; NPD) or isocaloric low protein diet (9% casein; LPD) restricted to one ovulatory cycle (3.5 days) prior to natural mating in female MF-1 mice. After mating, all females received NPD for the remainder of gestation and all offspring were litter size adjusted and fed standard chow. No difference in gestation length, litter size, sex ratio or postnatal growth was observed between treatments. Maternal LPD did, however, induce abnormal anxiety-related behaviour in open field activities in male and female offspring (P < 0.05). Maternal LPD offspring also exhibited elevated systolic blood pressure (SBP) in males at 9 and 15 weeks and in both sexes at 21 weeks (P < 0.05). Male LPD offspring hypertension was accompanied by attenuated arterial responsiveness in vitro to vasodilators acetylcholine and isoprenaline (P < 0.05). LPD female offspring adult kidneys were also smaller, but had increased nephron numbers (P < 0.05). Moreover, the relationship between SBP and kidney or heart size or nephron number was altered by diet treatment (P < 0.05). These data demonstrate the sensitivity of mouse maturing oocytes in vivo to maternal protein undernutrition and identify both behavioural and cardiovascular postnatal outcomes, indicative of adult disease. These outcomes probably derive from a direct effect of protein restriction, although indirect stress mechanisms may also be contributory. Similar and distinct postnatal outcomes were observed here compared with maternal LPD treatment during post-fertilization preimplantation development which may reflect the relative contribution of the paternal genome.
Figures
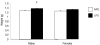

References
-
- Adamiak SJ, Mackie K, Watt RG, Webb R, Sinclair KD. Impact of nutrition on oocyte quality: cumulative effects of body composition and diet leading to hyperinsulinemia in cattle. Biol Reprod. 2005;73:918–926. - PubMed
-
- Amleh A, Dean J. Mouse genetics provides insight into folliculogenesis, fertilization and early embryonic development. Hum Reprod Update. 2002;8:395–403. - PubMed
-
- Angiolini E, Fowden A, Coan P, Sandovici I, Smith P, Dean W, Burton G, Tycko B, Reik W, Sibley C, Constancia M. Regulation of placental efficiency for nutrient transport by imprinted genes. Placenta. 2006;27(Suppl. A):S98–S102. - PubMed
-
- Armstrong DG, McEvoy TG, Baxter G, Robinson JJ, Hogg CO, Woad KJ, Webb R, Sinclair KD. Effect of dietary energy and protein on bovine follicular dynamics and embryo production in vitro: associations with the ovarian insulin-like growth factor system. Biol Reprod. 2001;64:1624–1632. - PubMed
-
- Bagby SP. Maternal nutrition, low nephron number, and hypertension in later life: pathways of nutritional programming. J Nutr. 2007;137:1066–1072. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/F007450/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BHF_/British Heart Foundation/United Kingdom
- G18784/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0100558/MRC_/Medical Research Council/United Kingdom
- U01 HD044635/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

