Response gene to complement 32 expression is induced by the luteinizing hormone (LH) surge and regulated by LH-induced mediators in the rodent ovary
- PMID: 18308847
- PMCID: PMC2408821
- DOI: 10.1210/en.2007-1129
Response gene to complement 32 expression is induced by the luteinizing hormone (LH) surge and regulated by LH-induced mediators in the rodent ovary
Abstract
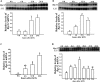
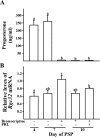
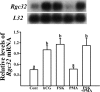
Response gene to complement 32 (Rgc32) has recently been suggested to be expressed in the ovary and regulated by RUNX1, a transcription factor in periovulatory follicles. In the present study, we determined the expression profile of the Rgc32 gene in the rodent ovary throughout the reproductive cycle and the regulatory mechanism(s) involved in Rgc32 expression during the periovulatory period. Northern blot and in situ hybridization analyses revealed the up-regulation of Rgc32 expression in periovulatory follicles. Rgc32 mRNA was also localized to newly forming corpora lutea (CL) and CL from previous estrous cycles. Further studies using hormonally induced luteal and luteolysis models revealed a transient increase in levels of Rgc32 mRNA at the time of functional regression of the CL. Next, the regulation of Rgc32 expression was investigated in vitro using rat preovulatory granulosa cells. The effect of human chorionic gonadotropin on Rgc32 expression was mimicked by forskolin, but not phorbol 12-myristate 13-acetate, and was mediated by the activation of progesterone receptors and the epidermal growth factor-signaling pathway. The mechanism by which RUNX1 regulates Rgc32 expression was investigated using chromatin immunoprecipitation and Rgc32 promoter-luciferase reporter assays. Data from these assays revealed direct binding of RUNX1 in the Rgc32 promoter region in vivo as well as the involvement of RUNX binding sites in the transactivation of the Rgc32 promoter in vitro. In summary, the present study demonstrated the spatial/temporal-specific expression of Rgc32 in the ovary, and provided evidence of LH-initiated and RUNX1-mediated expression of Rgc32 gene in luteinizing granulosa cells.
Figures






Similar articles
-
Luteinizing hormone-induced RUNX1 regulates the expression of genes in granulosa cells of rat periovulatory follicles.Mol Endocrinol. 2006 Sep;20(9):2156-72. doi: 10.1210/me.2005-0512. Epub 2006 May 4. Mol Endocrinol. 2006. PMID: 16675540 Free PMC article.
-
RUNX2 transcription factor regulates gene expression in luteinizing granulosa cells of rat ovaries.Mol Endocrinol. 2010 Apr;24(4):846-58. doi: 10.1210/me.2009-0392. Epub 2010 Mar 2. Mol Endocrinol. 2010. PMID: 20197312 Free PMC article.
-
Runt-related transcription factor 1 regulates luteinized hormone-induced prostaglandin-endoperoxide synthase 2 expression in rat periovulatory granulosa cells.Endocrinology. 2009 Jul;150(7):3291-300. doi: 10.1210/en.2008-1527. Epub 2009 Apr 2. Endocrinology. 2009. PMID: 19342459 Free PMC article.
-
Ovarian cell differentiation: a cascade of multiple hormones, cellular signals, and regulated genes.Recent Prog Horm Res. 1995;50:223-54. doi: 10.1016/b978-0-12-571150-0.50014-7. Recent Prog Horm Res. 1995. PMID: 7740159 Review.
-
Control of oocyte release by progesterone receptor-regulated gene expression.Nucl Recept Signal. 2009 Dec 31;7:e012. doi: 10.1621/nrs.07012. Nucl Recept Signal. 2009. PMID: 20087433 Free PMC article. Review.
Cited by
-
Interleukin-6: an autocrine regulator of the mouse cumulus cell-oocyte complex expansion process.Endocrinology. 2009 Jul;150(7):3360-8. doi: 10.1210/en.2008-1532. Epub 2009 Mar 19. Endocrinology. 2009. PMID: 19299453 Free PMC article.
-
RGC-32 is a novel regulator of the T-lymphocyte cell cycle.Exp Mol Pathol. 2015 Jun;98(3):328-37. doi: 10.1016/j.yexmp.2015.03.011. Epub 2015 Mar 11. Exp Mol Pathol. 2015. PMID: 25770350 Free PMC article.
-
The expression of CXCR4 is induced by the luteinizing hormone surge and mediated by progesterone receptors in human preovulatory granulosa cells.Biol Reprod. 2017 Jun 1;96(6):1256-1266. doi: 10.1093/biolre/iox054. Biol Reprod. 2017. PMID: 28595291 Free PMC article.
-
Prolactin signaling through the short isoform of the mouse prolactin receptor regulates DNA binding of specific transcription factors, often with opposite effects in different reproductive issues.Reprod Biol Endocrinol. 2009 Aug 24;7:87. doi: 10.1186/1477-7827-7-87. Reprod Biol Endocrinol. 2009. PMID: 19703295 Free PMC article.
-
Periovulatory expression of hyaluronan and proteoglycan link protein 1 (Hapln1) in the rat ovary: hormonal regulation and potential function.Mol Endocrinol. 2010 Jun;24(6):1203-17. doi: 10.1210/me.2009-0325. Epub 2010 Mar 25. Mol Endocrinol. 2010. PMID: 20339004 Free PMC article.
References
-
- Murphy BD 2000 Models of luteinization. Biol Reprod 63:2–11 - PubMed
-
- Robker RL, Richards JS 1998 Hormonal control of the cell cycle in ovarian cells: proliferation versus differentiation. Biol Reprod 59:476–482 - PubMed
-
- Stocco C, Telleria C, Gibori G 2007 The molecular control of corpus luteum formation, function, and regression. Endocr Rev 28:117–149 - PubMed
-
- Coffman JA 2003 Runx transcription factors and the developmental balance between cell proliferation and differentiation. Cell Biol Int 27:315–324 - PubMed

