Progesterone receptors and neural development: a gap between bench and bedside?
- PMID: 18308849
- PMCID: PMC2408811
- DOI: 10.1210/en.2008-0049
Progesterone receptors and neural development: a gap between bench and bedside?
Abstract
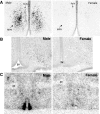
Despite a recent increase in the clinical use of progesterone in pregnant women and premature neonates, very little is understood about the potential role of this hormone and its receptors in neural development. Findings from rodent models indicate that the brain is indeed sensitive to progesterone during critical periods of development and maturation. Dramatic sex differences in progesterone receptor (PR) expression, in which males express higher levels of PR than females in specific regions, suggest that PR may play an important role in the sexual differentiation of brain and behavior and that the expression of PR may be one mechanism by which testicular hormones masculinize the brain. PR is also transiently expressed during fetal and neonatal development in areas of the brain associated with cognitive behaviors. PR protein and mRNA are expressed in pyramidal cell layers of perinatal cortex in an anatomically and developmentally specific manner, generating the intriguing hypothesis that progesterone is essential for normal cortical development. Basic research elucidating a potential role for progesterone and PR in developing brain is reviewed in light of the clinical use of this hormone. The necessity for future research integrating findings from the bench and the bedside is evident.
Figures



Similar articles
-
Sensorimotor development in neonatal progesterone receptor knockout mice.Dev Neurobiol. 2014 Jan;74(1):16-24. doi: 10.1002/dneu.22124. Epub 2013 Oct 7. Dev Neurobiol. 2014. PMID: 23983142
-
The many faces of progesterone: a role in adult and developing male brain.Front Neuroendocrinol. 2006 Sep;27(3):340-59. doi: 10.1016/j.yfrne.2006.07.003. Epub 2006 Oct 2. Front Neuroendocrinol. 2006. PMID: 17014900 Review.
-
Regulation of progesterone receptor expression by estradiol is dependent on age, sex and region in the rat brain.Endocrinology. 2008 Jun;149(6):3054-61. doi: 10.1210/en.2007-1133. Epub 2008 Feb 28. Endocrinology. 2008. PMID: 18308846 Free PMC article.
-
Progesterone receptors and the sexual differentiation of the medial preoptic nucleus.J Neurobiol. 2002 Apr;51(1):24-32. doi: 10.1002/neu.10040. J Neurobiol. 2002. PMID: 11920725
-
Progesterone receptors: form and function in brain.Front Neuroendocrinol. 2008 May;29(2):313-39. doi: 10.1016/j.yfrne.2008.02.001. Epub 2008 Feb 23. Front Neuroendocrinol. 2008. PMID: 18374402 Free PMC article. Review.
Cited by
-
Sex-specific effects of neonatal progestin receptor antagonism on juvenile social play behavior in rats.Behav Brain Funct. 2021 Nov 5;17(1):10. doi: 10.1186/s12993-021-00183-z. Behav Brain Funct. 2021. PMID: 34740365 Free PMC article.
-
Progesterone enhances learning and memory of aged wildtype and progestin receptor knockout mice.Neurosci Lett. 2010 Mar 12;472(1):38-42. doi: 10.1016/j.neulet.2010.01.051. Epub 2010 Feb 1. Neurosci Lett. 2010. PMID: 20117174 Free PMC article.
-
Elevated plasma corticosterone decreases yolk testosterone and progesterone in chickens: linking maternal stress and hormone-mediated maternal effects.PLoS One. 2011;6(8):e23824. doi: 10.1371/journal.pone.0023824. Epub 2011 Aug 23. PLoS One. 2011. PMID: 21886826 Free PMC article.
-
The ENDpoiNTs Project: Novel Testing Strategies for Endocrine Disruptors Linked to Developmental Neurotoxicity.Int J Mol Sci. 2020 Jun 1;21(11):3978. doi: 10.3390/ijms21113978. Int J Mol Sci. 2020. PMID: 32492937 Free PMC article.
-
Genomics of preterm birth.Cold Spring Harb Perspect Med. 2015 Feb 2;5(2):a023127. doi: 10.1101/cshperspect.a023127. Cold Spring Harb Perspect Med. 2015. PMID: 25646385 Free PMC article. Review.
References
-
- Ness A, Dias T, Damus K, Burd I, Berghella V 2006 Impact of the recent randomized trials on the use of progesterone to prevent preterm birth: a 2005 follow-up survey. Am J Obstet Gynecol 195:1174–1179 - PubMed
-
- Betrabet SS, Shikary ZK, Toddywalla VS, Toddywalla SP, Patel D, Saxena BN 1987 ICMR Task Force Study on hormonal contraception. Transfer of norethisterone (NET) and levonorgestrel (LNG) from a single tablet into the infant’s circulation through the mother’s milk. Contraception 35:517–522 - PubMed
-
- Toddywalla VS, Patel SB, Betrabet SS, Kulkarni RD, Saxena BN 1995 Is time-interval between mini-pill ingestion and breastfeeding essential? Contraception 51:193–195 - PubMed
-
- Gainer E, Massai R, Lillo S, Reyes V, Forcelledo ML, Caviedes R, Villarroel C, Bouyer J 2007 Levonorgestrel pharmacokinetics in plasma and milk of lactating women who take 1.5 mg for emergency contraception. Hum Reprod 22:1578 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

