Oocyte-specific overexpression of mouse bone morphogenetic protein-15 leads to accelerated folliculogenesis and an early onset of acyclicity in transgenic mice
- PMID: 18308851
- PMCID: PMC2408818
- DOI: 10.1210/en.2007-1550
Oocyte-specific overexpression of mouse bone morphogenetic protein-15 leads to accelerated folliculogenesis and an early onset of acyclicity in transgenic mice
Abstract
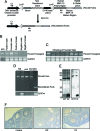
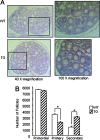
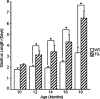
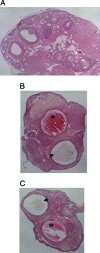
Whereas mutations in the bmp15 gene cause infertility in ewes and women due to defects in folliculogenesis, most defects in female mice lacking bone morphogenetic protein (BMP)-15 are confined to the ovulation process, supportive of the observation that functional mouse BMP-15 is barely detected in oocytes in vivo until after the LH surge. In addition, the mouse BMP-15 proprotein is not processed into the functional mature protein in transfected cells. However, a chimeric protein consisting of the human proregion, human cleavage site, and mouse mature region (termed hhmBMP-15) is processed and the mature protein secreted. To study the role of BMP-15 in folliculogenesis, we generated transgenic mice overexpressing hhmBMP-15, exclusively in oocytes during folliculogenesis and confirmed the overexpression of mouse BMP-15 mature protein. Immature transgenic mice exhibited accelerated follicle growth with decreased primary follicles and an increase in secondary follicles. Granulosa cells of immature mice displayed an increased mitotic index and decreased FSH receptor mRNA expression. Adult mice had normal litter sizes but an increased number of atretic antral follicles. Interestingly, aging mice exhibited an early onset of acyclicity marked by increased diestrus length and early occurrence of constant diestrus. These findings indicate the role of BMP-15 in vivo in promoting follicle growth and preventing follicle maturation, resulting in an early decline in the ovarian reserve of transgenic mice. Therefore, the lack of mouse BMP-15 during early folliculogenesis in the wild-type mice may be relevant to their polyovulatory nature as well as the preservation of ovarian function as the mice age.
Figures





Similar articles
-
The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders.Hum Reprod Update. 2014 Nov-Dec;20(6):869-83. doi: 10.1093/humupd/dmu036. Epub 2014 Jun 30. Hum Reprod Update. 2014. PMID: 24980253 Review.
-
Oocyte bone morphogenetic protein 15, but not growth differentiation factor 9, is increased during gonadotropin-induced follicular development in the immature mouse and is associated with cumulus oophorus expansion.Biol Reprod. 2006 Dec;75(6):836-43. doi: 10.1095/biolreprod.106.055574. Epub 2006 Aug 30. Biol Reprod. 2006. PMID: 16943361
-
Molecular biology and physiological role of the oocyte factor, BMP-15.Mol Cell Endocrinol. 2005 Apr 29;234(1-2):67-73. doi: 10.1016/j.mce.2004.10.012. Mol Cell Endocrinol. 2005. PMID: 15836954 Review.
-
Inhibition of oocyte growth factors in vivo modulates ovarian folliculogenesis in neonatal and immature mice.Reproduction. 2010 Mar;139(3):587-98. doi: 10.1530/REP-09-0391. Epub 2009 Dec 10. Reproduction. 2010. PMID: 20007639
-
A unique preovulatory expression pattern plays a key role in the physiological functions of BMP-15 in the mouse.Proc Natl Acad Sci U S A. 2006 Jul 11;103(28):10678-83. doi: 10.1073/pnas.0600507103. Epub 2006 Jul 3. Proc Natl Acad Sci U S A. 2006. PMID: 16818886 Free PMC article.
Cited by
-
Novel variants in women with premature ovarian function decline identified via whole-exome sequencing.J Assist Reprod Genet. 2020 Oct;37(10):2487-2502. doi: 10.1007/s10815-020-01919-y. Epub 2020 Aug 13. J Assist Reprod Genet. 2020. PMID: 32789750 Free PMC article.
-
BMP15 Modulates the H19/miR-26b/SMAD1 Axis Influences Yak Granulosa Cell Proliferation, Autophagy, and Apoptosis.Reprod Sci. 2023 Apr;30(4):1266-1280. doi: 10.1007/s43032-022-01051-5. Epub 2022 Sep 7. Reprod Sci. 2023. PMID: 36071342
-
Association between BMP15 Gene Polymorphism and Reproduction Traits and Its Tissues Expression Characteristics in Chicken.PLoS One. 2015 Nov 17;10(11):e0143298. doi: 10.1371/journal.pone.0143298. eCollection 2015. PLoS One. 2015. PMID: 26574748 Free PMC article.
-
Overexpression of Uromodulin-like1 accelerates follicle depletion and subsequent ovarian degeneration.Cell Death Dis. 2012 Nov 29;3(11):e433. doi: 10.1038/cddis.2012.169. Cell Death Dis. 2012. PMID: 23190605 Free PMC article.
-
Multigenerational obesity-induced perturbations in oocyte-secreted factor signalling can be ameliorated by exercise and nicotinamide mononucleotide.Hum Reprod Open. 2018 May 29;2018(3):hoy010. doi: 10.1093/hropen/hoy010. eCollection 2018. Hum Reprod Open. 2018. PMID: 30895251 Free PMC article.
References
-
- Moore RK, Erickson GF, Shimasaki S 2004 Are BMP-15 and GDF-9 primary determinants of ovulation quota in mammals? Trends Endocrinol Metab 15:356–361 - PubMed
-
- Galloway SM, McNatty KP, Cambridge LM, Laitinen MPE, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, Beattie AE, Davis GH, Ritvos O 2000 Mutations in an oocyte-derived growth factor gene (bmp15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet 25:279–283 - PubMed
-
- Hanrahan JP, Gregan SM, Mulsant P, Mullen M, Davis GH, Powell R, Galloway SM 2004 Mutations in the genes for oocyte-derived growth factors gdf9 and bmp15 are associated with both increased ovulation rate and sterility in cambridge and belclare sheep (ovis aries). Biol Reprod 70:900–909 - PubMed
-
- McNatty KP, Juengel JL, Wilson T, Galloway SM, Davis GH 2001 Genetic mutations influencing ovulation rate in sheep. Reprod Fertil Dev 13:549–555 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

