T cell expression of MyD88 is required for resistance to Toxoplasma gondii
- PMID: 18308927
- PMCID: PMC2268781
- DOI: 10.1073/pnas.0706663105
T cell expression of MyD88 is required for resistance to Toxoplasma gondii
Abstract
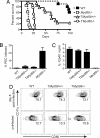
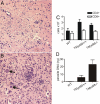
Resistance to Toxoplasma gondii depends on dendritic cells to recognize this pathogen and secrete IL-12, in turn promoting IFN-gamma production from responding T cells. The adaptor protein, myeloid differentiation primary-response gene 88 (MyD88), is important for most Toll-like receptor (TLR) signaling, as well as IL-1R/IL-18R signals. There is considerable evidence that MyD88 is required for the innate sensing of T. gondii and IL-12 responses. Although Myd88(-/-) mice challenged with T. gondii have defective IL-12 and Th1 effector responses and succumb to disease, administration of IL-12 to Myd88(-/-) mice partially restores the Th1 response and yet fails to prolong survival. This finding suggested that MyD88 may mediate signals within T cells important for resistance to this pathogen. To evaluate the role of MyD88 in T cells under noncompetitive conditions, bone marrow chimeras were generated, in which the T cells lacked MyD88, but MyD88-dependent innate immune responses were intact. Upon challenge with T. gondii, these chimeric mice were more susceptible to disease, developing severe toxoplasmic encephalitis and succumbing within 30 days. Splenocytes and brain mononuclear cells isolated from infected chimeric mice produced less IFN-gamma when cultured with a T. gondii-derived antigen. The increase in susceptibility observed was independent of signals via the IL-1R and IL-18R, suggesting a role for TLRs in MyD88-mediated T cell responses to T. gondii. These observations show that, in addition to a role for MyD88 in innate responses, T cell expression of MyD88 is necessary for prolonged resistance to a pathogen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. - PubMed
-
- O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. - PubMed
-
- Seki E, et al. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J Immunol. 2002;169:3863–3868. - PubMed
-
- Edelson BT, Unanue ER. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: No role for either in macrophage listericidal activity. J Immunol. 2002;169:3869–3875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

