A trans-Golgi network golgin is required for the regulated secretion of TNF in activated macrophages in vivo
- PMID: 18308930
- PMCID: PMC2265200
- DOI: 10.1073/pnas.0800137105
A trans-Golgi network golgin is required for the regulated secretion of TNF in activated macrophages in vivo
Abstract
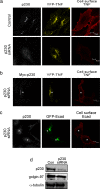
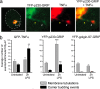
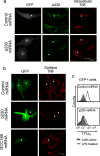
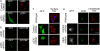
The transmembrane precursor of tumor necrosis factor-alpha (TNF) exits the trans-Golgi network (TGN) in tubular carriers for subsequent trafficking and delivery to the cell surface; however, the molecular machinery responsible for Golgi export is unknown. We previously reported that members of the TGN golgin family are associated with subdomains and tubules of the TGN. Here, we show that the TGN golgin, p230/golgin-245 (p230), is essential for intracellular trafficking and cell surface delivery of TNF in transfected HeLa cells and activated macrophages. Live-cell imaging revealed that TNF transport from the TGN is mediated selectively by tubules and carriers marked by p230. Significantly, LPS activation of macrophages resulted in a dramatic increase of p230-labeled tubules and carriers emerging from the TGN, indicating that macrophages up-regulate the transport pathway for TNF export. Depletion of p230 in LPS-stimulated macrophages reduced cell surface delivery of TNF by >10-fold compared with control cells. To determine whether p230 depletion blocked TNF secretion in vivo, we generated retrogenic mice expressing a microRNA-vector to silence p230. Bone-marrow stem cells were transduced with recombinant retrovirus containing microRNA constructs and transplanted into irradiated recipients. LPS-activated peritoneal macrophages from p230 miRNA retrogenic mice were depleted of p230 and had dramatically reduced levels of cell surface TNF. Overall, these studies have identified p230 as a key regulator of TNF secretion and have shown that LPS activation of macrophages results in increased Golgi carriers for export. Also, we have demonstrated a previously undescribed approach to control cytokine secretion by the specific silencing of trafficking machinery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hume DA. The mononuclear phagocyte system. Curr Opin Immunol. 2006;18:49–53. - PubMed
-
- Beutler BA. The role of tumor necrosis factor in health and disease. J Rheumatol. 1999;57:16–21. - PubMed
-
- Siddiqui MA, Scott LJ. Infliximab: a review of its use in Crohn's disease and rheumatoid arthritis. Drugs. 2005;65:2179–2208. - PubMed
-
- Murray RZ, Kay JG, Sangermani DG, Stow JL. A role for the phagosome in cytokine secretion. Science. 2005;310:1492–1495. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

